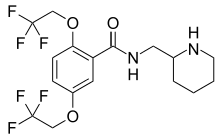
Flecainide
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Tambocor |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608040 |
| Pregnancy category |
|
| ATC code | C01BC04 (WHO) |
| Pharmacokinetic data | |
| Bioavailability | 95% |
| Protein binding | 40% |
| Metabolism | CYP2D6 (limited) |
| Biological half-life | 20 hours (range 12-27 hours) |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
54143-55-4 |
| PubChem (CID) | 3356 |
| IUPHAR/BPS | 2560 |
| DrugBank |
DB01195 |
| ChemSpider |
3239 |
| UNII |
K94FTS1806 |
| KEGG |
D07962 |
| ChEBI |
CHEBI:75984 |
| ChEMBL |
CHEMBL652 |
| ECHA InfoCard | 100.211.334 |
| Chemical and physical data | |
| Formula | C17H20F6N2O3 |
| Molar mass | 414.343 g/mol |
| 3D model (Jmol) | Interactive image |
| Chirality | Racemic mixture |
| |
| |
| | |
Flecainide acetate (/flɛˈkeɪnaɪd/ US dict: fle·kā′·nīd) is a class Ic antiarrhythmic agent used to prevent and treat tachyarrhythmias (abnormal fast rhythms of the heart). It is used to treat a variety of cardiac arrhythmias including paroxysmal atrial fibrillation (episodic irregular heartbeat originating in the upper chamber of the heart), paroxysmal supraventricular tachycardia (episodic rapid but regular heartbeat originating in the atrium), and ventricular tachycardia (rapid rhythms of the lower chambers of the heart). Flecainide works by regulating the flow of sodium in the heart, causing prolongation of the cardiac action potential.
Flecainide is sold under the trade name Tambocor (manufactured by 3M pharmaceuticals). Flecainide went off-patent on February 10, 2004. In addition to being marketed as Tambocor, it is also available in generic version and under the trade names Almarytm, Apocard, Ecrinal, and Flécaine.
Medical uses
Flecainide is used in the treatment of many types of supraventricular tachycardias, including AV nodal re-entrant tachycardia (AVNRT) and Wolff-Parkinson-White syndrome (WPW). This is because of the action of flecainide on the His-Purkinje system.
It also has limited use in the treatment of certain forms of ventricular tachycardia (VT). In particular, flecainide has been useful in the treatment of ventricular tachycardias that are not in the setting of an acute ischemic event. It has use in the treatment of right ventricular outflow tract (RVOT) tachycardia[1] and in the suppression of arrhythmias in arrhythmogenic right ventricular dysplasia (ARVD).[2] Studies (notably the Cardiac Arrhythmia Suppression Trial) have shown an increased mortality when flecainide is used to suppress ventricular extrasystoles in the setting of acute myocardial infarction.[3][4]
In individuals suspected of having the Brugada syndrome, the administration of flecainide may help reveal the ECG findings that are characteristic of the disease process. This may help make the diagnosis of the disease in equivocal cases.[5]
Flecainide has been introduced into the treatment of arrhythmias in the pediatric population.
Side effects
Results of a medical study known as the Cardiac Arrhythmia Suppression Trial (CAST) demonstrated that patients with structural heart disease (such as a history of MI (heart attack), or left ventricular dysfunction) and also patients with ventricular arrhythmias, should not take this drug. The results were so significant that the trial was stopped early and preliminary results were published.[6]
The dose may need to be adjusted in certain clinical scenarios. As with all other antiarrhythmic agents, there is a risk of proarrhythmia associated with the use of flecainide. This risk is probably increased when flecainide is co-administered with other class Ic antiarrhythmics, such as encainide. The risk of proarrhythmia may also be increased by hypokalemia.[7] The risk of proarrhythmia is not necessarily associated with the length of time an individual is taking flecainide, and cases of late proarrhythmia have been reported.[8] Because of the role of both the liver and the kidneys in the elimination of flecainide, the dosing of flecainide may need to be adjusted in individuals who develop either liver failure or renal failure.
Because of the negative inotropic effects of flecainide, it should be used with caution in individuals with depressed ejection fraction, and may worsen congestive heart failure in these individuals. It should be avoided in people with ischaemic heart disease and the elderly.[9]
As with all class I antiarrhythmic agents, Flecainide increases the capture thresholds of pacemakers.[10]
Cardiac toxicity
Due to the narrow therapeutic index of flecainide, physicians should be alert for signs of toxicity before life-threatening arrhythmias occur like torsades de pointes. While the toxic effects of flecainide are closely related to the plasma levels of the drug,[11] it is unfeasible to check the plasma concentration in an individual on a regular basis.
Signs of flecainide toxicity include marked prolongation of the PR interval and widening of the QRS duration on the surface ECG. There may be signs and symptoms attributable to overt heart failure secondary to sudden decreased myocardial contractility.
Treatment
Treatment of flecainide cardiac toxicity involves increasing the excretion of flecainide, blocking its effects in the heart, and (rarely) institution of cardiovascular support to avoid impending lethal arrhythmias. Modalities that have had success include administration of a beta-sympathomimetic agent,[11] and administration of a sodium load[11](often in the form of hypertonic sodium bicarbonate). Placing the individual on cardiopulmonary bypass support may be necessary in order to temporarily remove the need for a beating heart and to increase blood flow to the liver.[12][13]
Lung toxicity
Flecainide has a very high affinity for lung tissue [14] and is associated with drug-induced interstitial lung disease.[15][16][17][18][19]
Mechanism of action
Flecainide works by blocking the Nav1.5 sodium channel in the heart, slowing the upstroke of the cardiac action potential.[20] This thereby slows conduction of the electrical impulse within the heart, i.e. it "reduces excitability". The greatest effect is on the His-Purkinje system and ventricular myocardium. The effect of flecainide on the ventricular myocardium causes decreased contractility of the muscle, which leads to a decrease in the ejection fraction.
The effect of flecainide on the sodium channels of the heart increases as the heart rate increases; This is known as use-dependence and is why that flecainide is useful to break a tachyarrhythmia.[21]
Flecainide also inhibits ryanodine receptor 2 (RyR2),[22] a major regulator of sarcoplasmic release of stored calcium ions. It can reduce calcium sparks and thus arrhythmogenic calcium waves in the heart.[23] While Flecainide therapy has been shown to suppress ventricular arrhythmias in patients with catecholaminergic polymorphic ventricular tachycardia (CPVT) and mouse models of this disease, the relative contribution from the inhibition of sodium channels and of RyR2 in this effect on CPVT is unclear.[24]
Metabolism and drug interactions
Flecainide has high bioavailability after an oral dose,[25] meaning that most of the drug that is ingested will enter the systemic blood stream. Peak serum concentrations can be seen 1 to 6 hours after ingestion of an oral dose. While the plasma half-life is about 20 hours, it is quite variable, and can range from 12 to 27 hours.[26] During oral loading with flecainide, a steady state equilibrium is typically achieved in 3 to 5 days.
The majority of flecainide is eliminated by the kidneys, with the remainder metabolized by the cytochrome P450 2D6 isoenzyme in the liver.[27] Therefore, alterations in renal function or urine pH will greatly affect the elimination of flecainide, as more is eliminated by the kidney than by the hepatic route.
Because of the dual elimination routes of flecainide and its tendency to decrease myocardial contractility,[9] flecainide interacts with numerous pharmaceuticals and can potentiate the effects of other myocardial depressants and AV node blocking agents. In addition, flecainide can decrease the metabolism or elimination of many (but not all) agents that use the cytochrome P450 enzyme system.
A full list of drug interactions with flecainide can be obtained from the manufacturer. Some important drug interactions with flecainide include:
- Alcohol - may further depress normal heart function.
- Amiodarone - inhibits cytochrome P450 2D6 and may increase flecainide levels
- Cimetidine - increases flecainide levels by 30% and half-life by 10%
- Digoxin - may increase digoxin levels
- Paroxetine - increased effect of both drugs.
- Propafenone - increased effect of both drugs and increased risk of toxicity.
- Quinidine - inhibits cytochrome P450 2D6 and may increase flecainide levels
Long-term effects
In the long term, flecainide seems to be safe in patients with a healthy heart with no signs of left ventricular hypertrophy, ischemic heart disease or heart failure.[28]
References
- ↑ Gill J, Mehta D, Ward D, Camm A (1992). "Efficacy of flecainide, sotalol, and verapamil in the treatment of right ventricular tachycardia in patients without overt cardiac abnormality". Br Heart J. 68 (4): 392–7. doi:10.1136/hrt.68.10.392. PMC 1025139
 . PMID 1449923.
. PMID 1449923. - ↑ Sakurada H, Hiyoshi Y, Tejima T, Yanase O, Tokuyasu Y, Watanabe K, Motomiya T, Sugiura M, Hiraoka M (1990). "[Effects of oral flecainide treatment of refractory tachyarrhythmias]". Kokyu to Junkan. 38 (5): 471–6. PMID 2115193.
- ↑ Echt D, Liebson P, Mitchell L, Peters R, Obias-Manno D, Barker A, Arensberg D, Baker A, Friedman L, Greene H (1991). "Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial". N Engl J Med. 324 (12): 781–8. doi:10.1056/NEJM199103213241201. PMID 1900101.
- ↑ Greenberg H, Dwyer E, Hochman J, Steinberg J, Echt D, Peters R (1995). "Interaction of ischaemia and encainide/flecainide treatment: a proposed mechanism for the increased mortality in CAST I". Br Heart J. 74 (6): 631–5. doi:10.1136/hrt.74.6.631. PMC 484119
 . PMID 8541168.
. PMID 8541168. - ↑ Gasparini M, Priori S, Mantica M, Napolitano C, Galimberti P, Ceriotti C, Simonini S (2003). "Flecainide test in Brugada syndrome: a reproducible but risky tool". Pacing Clin Electrophysiol. 26 (1 Pt 2): 338–41. doi:10.1046/j.1460-9592.2003.00045.x. PMID 12687841.
- ↑ Cardiac Arrhythmia Suppression Trial (CAST) Investigators (1989). "Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction.". N Engl J Med. 321 (6): 406–412. doi:10.1056/NEJM198908103210629. PMID 2473403.
- ↑ Ohki R, Takahashi M, Mizuno O, Fujikawa H, Mitsuhashi T, Katsuki T, Ikeda U, Shimada K (2001). "Torsades de pointes ventricular tachycardia induced by mosapride and flecainide in the presence of hypokalemia". Pacing Clin Electrophysiol. 24 (1): 119–21. doi:10.1046/j.1460-9592.2001.00119.x. PMID 11227957.
- ↑ Morganroth J (1992). "Early and late proarrhythmia from antiarrhythmic drug therapy". Cardiovasc Drugs Ther. 6 (1): 11–4. doi:10.1007/BF00050910. PMID 1533532.
- 1 2 Santinelli V, Arnese M, Oppo I, Matarazzi C, Maione S, Palma M, Giunta A (1993). "Effects of flecainide and propafenone on systolic performance in subjects with normal cardiac function". Chest. 103 (4): 1068–73. doi:10.1378/chest.103.4.1068. PMID 8131440.
- ↑ Fornieles-Pérez H, Montoya-García M, Levine P, Sanz O (2002). "Documentation of acute rise in ventricular capture thresholds associated with flecainide acetate". Pacing Clin Electrophysiol. 25 (5): 871–2. doi:10.1046/j.1460-9592.2002.00871.x. PMID 12049386.
- 1 2 3 Winkelmann B, Leinberger H (1987). "Life-threatening flecainide toxicity. A pharmacodynamic approach". Annals of Internal Medicine. 106 (6): 807–14. doi:10.7326/0003-4819-106-6-807. PMID 3107447.
- ↑ Corkeron M, van Heerden P, Newman S, Dusci L (1999). "Extracorporeal circulatory support in near-fatal flecainide overdose". Anaesth Intensive Care. 27 (4): 405–8. PMID 10470398.
- ↑ Yasui R, Culclasure T, Kaufman D, Freed C (1997). "Flecainide overdose: is cardiopulmonary support the treatment?". Annals of Emergency Medicine. 29 (5): 680–2. doi:10.1016/S0196-0644(97)70257-9. PMID 9140253.
- ↑ Latini R, Cavalli A, Maggioni AP, Volpi A (December 1987). "Flecainide distribution in human tissues". 24 (6): 820–2. doi:10.1111/j.1365-2125.1987.tb03252.x. PMC 1386410
 . PMID 3125854.
. PMID 3125854. - ↑ Ozkan M, Dweik RA, Ahmad M (September 2001). "Drug-induced lung disease". 68 (9): 782–5, 789–95. doi:10.3949/ccjm.68.9.782. PMID 11563482.
- ↑ P Camus, A Fanton, P Bonniaud, C Camus et al. "Interstitial lung disease induced by drugs and radiation. Respiration 2004; 71:301–326 doi:10.1159/000079633 PMID 15316202
- ↑ S Pesenti, D Lauque, G Daste, V Boulay et al. "Diffuse Infiltrative Lung Disease Associated with Flecainide. Respiration 2002; 69:182–185 doi:10.1159/000056325 PMID 11961436
- ↑ Haas M, Pérault MC, Bonnefoy P, Rodeau F, Caron F (2001). "[Interstitial pneumopathy due to flecainide]". 30 (21): 1062. PMID 11471279.
- ↑ Robain A, Perchet H, Fuhrman C (February 2000). "Flecainide-associated pneumonitis with acute respiratory failure in a patient with the LEOPARD syndrome". 55 (1): 45–7. doi:10.2143/ac.55.1.2005718. PMID 10707759.
- ↑ Ramos E, O'leary M (2004). "State-dependent trapping of flecainide in the cardiac sodium channel". J Physiol. 560 (Pt 1): 37–49. doi:10.1113/jphysiol.2004.065003. PMC 1665201
 . PMID 15272045.
. PMID 15272045. - ↑ Wang Z, Fermini B, Nattel S (1993). "Mechanism of flecainide's rate-dependent actions on action potential duration in canine atrial tissue". J Pharmacol Exp Ther. 267 (2): 575–81. PMID 8246130.
- ↑ Mehra D, Imtiaz MS, van Helden DF, Knollmann BC, Laver DR (2014). "Multiple modes of ryanodine receptor 2 inhibition by flecainide". Molecular Pharmacology. 86 (6): 696–706. doi:10.1124/mol.114.094623. PMID 25274603.
- ↑ Hilliard FA, Steele DS, Laver D, Yang Z, Le Marchand SJ, Chopra N, Piston DW, Huke S, Knollmann BC (2010). "Flecainide inhibits arrhythmogenic Ca2+ waves by open state block of ryanodine receptor Ca2+ release channels and reduction of Ca2+ spark mass". Journal of Molecular and Cellular Cardiology. 48 (2): 293–301. doi:10.1016/j.yjmcc.2009.10.005. PMC 2813417
 . PMID 19835880.
. PMID 19835880. - ↑ Smith GL, MacQuaide N (2015). "The direct actions of flecainide on the human cardiac ryanodine receptor: keeping open the debate on the mechanism of action of local anesthetics in CPVT". Circulation Research. 116 (8): 1284–6. doi:10.1161/CIRCRESAHA.115.306298. PMID 25858058.
- ↑ Smith G (1985). "Flecainide: a new class Ic antidysrhythmic". Drug Intell Clin Pharm. 19 (10): 703–7. PMID 3902429.
- ↑ Padrini R, Piovan D, Busa M, al-Bunni M, Maiolino P, Ferrari M (1993). "Pharmacodynamic variability of flecainide assessed by QRS changes". Clin Pharmacol Ther. 53 (1): 59–64. doi:10.1038/clpt.1993.9. PMID 8422742.
- ↑ Haefeli W, Bargetzi M, Follath F, Meyer U (1990). "Potent inhibition of cytochrome P450IID6 (debrisoquin 4-hydroxylase) by flecainide in vitro and in vivo". J Cardiovasc Pharmacol. 15 (5): 776–9. doi:10.1097/00005344-199005000-00013. PMID 1692938.
- ↑ Aliot E, Capucci A, Crijns HJ, Goette A, Tamargo J. Twenty-five years in the making: flecainide is safe and effective for the management of atrial fibrillation. Eurospace 2011 13:161-173