VG (nerve agent)
 | |
 | |
| Names | |
|---|---|
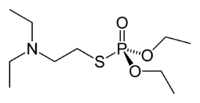
| IUPAC name
O,O-Diethyl S-[2-(diethylamino)ethyl] phosphorothioate | |
| Identifiers | |
| 78-53-5 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 6294 |
| PubChem | 6542 |
| |
| |
| Properties | |
| C10H24NO3PS | |
| Molar mass | 269.34 g·mol−1 |
| Boiling point | 110 °C (230 °F; 383 K) at 0.2 mmHg |
| Vapor pressure | 0.01 mmHg at 80 °C |
| Hazards | |
| NFPA 704 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
VG (IUPAC name: O,O-diethyl S-[2-(diethylamino)ethyl] phosphorothioate) (also called Amiton or Tetram) is a "V-series" nerve agent chemically similar to the better-known VX nerve agent. Tetram is the common Russian name for the substance. Amiton was the trade name for the substance when it was marketed as an insecticide by ICI in the mid-1950s.
With a toxicity of about 1/10 that of VX, i.e. similar to that of sarin,[1] it is now considered too dangerous for use in agriculture[2] but unlike other nerve agents it is classified under Schedule 2 of the Chemical Weapons Convention rather than the more restrictive Schedule 1. It is thought that North Korea may have military stockpiles of this chemical .[3]
During the early 1950s at least three chemical companies working on organo-phosphorus insecticides independently discovered the amazing toxicity of these chemicals.[4] In 1952, Dr. Ranajit Ghosh, a chemist working for ICI at their Plant Protection Laboratories was investigating the potential of organophosphate esters of substituted aminoethanethiols for use as pesticides. Like the earlier German investigators of organophosphates in the late 1930s who had discovered the G-series nerve agents, Dr. Ghosh discovered that their action on cholinesterase made them effective pesticides. One of them, Amiton, was described in a 1955 paper by Ghosh and another chemist, J. F. Newman, as being particularly effective against mites.[4] It was brought to market as an insecticide by the company in 1954 but was subsequently withdrawn as too toxic.[5]
The toxicity of these substances had not passed unnoticed by the British Government, as some of the compounds had already been sent to their research facility at Porton Down for evaluation. Some of the chemicals from this class of compounds formed a new group of nerve agents called V Agents. The British Government unilaterally renounced chemical and biological weapons in 1956, although in 1958 traded their research on VX technology with the United States Government in exchange for information on thermonuclear weapons. The US then went into production of large amounts of the chemically similar, but much more toxic VX in 1961.[6]
It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.[7]
References
- ↑ "Summary of CWC-Schedules and their Relevance to Chemical Warfare" (PDF). Australia's National Authority for the Chemical Weapons Convention. Retrieved 2006-10-07.
- ↑ Theodore Karasik. "Toxic Warfare" (PDF). RAND. Retrieved 2006-10-07.
- ↑ "North Korea Profile Chemical Agents VG (Amiton, Tetram)". Nuclear Threat Initiative. Retrieved 2006-10-07.
- 1 2 "Nerve Agents - Lethal organo-phosphorus compounds inhibiting cholinesterase". Organisation for the Prohibition of Chemical Weapons website. Retrieved 2006-10-07.
- ↑ "Nerve Agents: General". The site for information about chemical and biological weapons for emergency, safety and security personnel. Retrieved 2006-10-07.
- ↑ "A Short History of the Development of Nerve Gases". Mitrek Systems. Archived from the original on November 12, 2006. Retrieved 2006-10-07.
- ↑ "40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities" (PDF) (July 1, 2008 ed.). Government Printing Office. Retrieved October 29, 2011.
