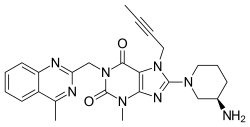
Linagliptin
 | |
| Clinical data | |
|---|---|
| Pronunciation | LIN-a-GLIP-tin |
| Trade names | Tradjenta, Trajenta |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a611036 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth (tablets) |
| ATC code | A10BH05 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~30% (Tmax = 1.5 hours) |
| Protein binding | 75–99% (concentration-dependent) |
| Metabolism | Minimal (~10% metabolized) |
| Metabolites | Pharmacologically inactive |
| Biological half-life | ~12 hours |
| Excretion | Feces (80%), urine (5%)[1] |
| Identifiers | |
| |
| CAS Number |
668270-12-0 |
| PubChem (CID) | 10096344 |
| IUPHAR/BPS | 6318 |
| ChemSpider |
8271879 |
| UNII |
3X29ZEJ4R2 |
| KEGG |
D09566 |
| ChEBI |
CHEBI:68610 |
| ChEMBL |
CHEMBL237500 |
| Chemical and physical data | |
| Formula | C25H28N8O2 |
| Molar mass | 472.54 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Linagliptin (INN,[2] previously known as BI-1356, marketed under trade names Tradjenta (U.S.) and Trajenta (worldwide)) is a dipeptidyl peptidase-4 inhibitor developed by Boehringer Ingelheim for treatment of diabetes mellitus type 2.
Once-daily linagliptin was approved by the U.S. Food and Drug Administration (FDA) on 2 May 2011 for treatment of type 2 diabetes.[3] It is being marketed by Boehringer Ingelheim and Lilly.
Medical uses
Results in 2010 from a Phase III clinical trial of linagliptin showed that the drug can effectively reduce blood sugar.[4]
Side effects
Linagliptin may cause severe joint pain.[1][5]
Mechanism of action
Linagliptin belongs to a class of drugs called DPP-4 inhibitors. They work by increasing hormones that stimulate pancreas to produce more insulin and stimulate your liver to produce less glucose. In other words, linagliptin, along with diet and exercise, can help the body produce more insulin and less sugar. Managing blood sugar can mean a lower HbA1c — that's an important goal for anyone with type 2 diabetes.
See also
References
- 1 2 "Tradjenta (linagliptin) Tablets. Full Prescribing Information" (PDF). Boehringer Ingelheim Pharmaceuticals, Inc. Ridgefield, CT 06877 USA. Retrieved 10 November 2016.
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary names: List 61" (PDF). World Health Organization. p. 66. Retrieved 10 November 2016.
- ↑ "FDA Approves Type 2 Diabetes Drug from Boehringer Ingelheim and Lilly". 3 May 2011.
- ↑ "Four Phase III Trials Confirm Benefits of BI's Oral, Once-Daily Type 2 Diabetes Therapy". Genetic Engineering & Biotechnology News. 28 June 2010.
- ↑ "DPP-4 Inhibitors for Type 2 Diabetes: Drug Safety Communication - May Cause Severe Joint Pain". FDA. 2015-08-28. Retrieved 1 September 2015.
- H. Spreitzer (September 1, 2008). "Neue Wirkstoffe - BI-1356". Österreichische Apothekerzeitung (in German) (18/2008): 918.
- Wang, Y, Serradell, N, Rosa, E, Castaner, R (2008). "BI-1356". Drugs of the Future. 33 (6): 473–477. doi:10.1358/dof.2008.033.06.1215244.
External links
| Wikimedia Commons has media related to Linagliptin. |
- Tradjenta official website (United States)
- Trajenta (Australia)
- Trajenta (Canada)
- Trajenta (European Union)
- Trajenta (India)