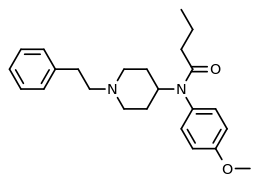
4-Methoxybutyrfentanyl
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | none |
| PubChem (CID) | 118796493 |
| ChemSpider | 52085457 |
| UNII |
Y72Z69R7K3 |
| Chemical and physical data | |
| Formula | C24H32N2O2 |
| Molar mass | 380.53 g·mol−1 |
| 3D model (Jmol) | Interactive image |
| |
| |
4-Methoxybutyrfentanyl (also known as 4-MeO-BF) is an opioid analgesic that is an analog of butyrfentanyl and has been sold online as a designer drug.[1][2][3]
Side effects
Side effects of fentanyl analogs are similar to those of fentanyl itself, which include itching, nausea and potentially serious respiratory depression, which can be life-threatening. Fentanyl analogs have killed hundreds of people throughout Europe and the former Soviet republics since the most recent resurgence in use began in Estonia in the early 2000s, and novel derivatives continue to appear.[4]
Life-threatening adverse reactions have been observed.[5]
Legal status
4-Methoxybutyrfentanyl is illegal in Sweden as of 26. January 2016.[6]
See also
- 3-Methylbutyrfentanyl
- 3-Methylfentanyl
- 4-Fluorobutyrfentanyl
- α-Methylfentanyl
- Acetylfentanyl
- Furanylfentanyl
- List of fentanyl analogues
References
- ↑ "4-MeO-Butyrfentanyl". Cayman Chemical.
- ↑ "4-Methoxybutyrfentanyl". New Synthetic Drugs Database.
- ↑ "ANALYTICAL REPORT - 4-MeO-BF" (PDF). European project RESPONSE.
- ↑ Jane Mounteney; Isabelle Giraudon; Gleb Denissov; Paul Griffiths (July 2015). "Fentanyls: Are we missing the signs? Highly potent and on the rise in Europe.". International Journal of Drug Policy. 26 (7): 626–631. doi:10.1016/j.drugpo.2015.04.003. PMID 25976511.
- ↑ Anders Helander; Matilda Bäckberg; Olof Beck (April 2016). "Intoxications involving the fentanyl analogs acetylfentanyl, 4-methoxybutyrfentanyl and furanylfentanyl: results from the Swedish STRIDA project". Clinical Toxicology. 54 (4): 324–332. doi:10.3109/15563650.2016.1139715. PMID 26850293.
- ↑ "31 nya ämnen kan klassas som narkotika eller hälsofarlig vara" (in Swedish). Folkhälsomyndigheten. November 2015.
This article is issued from Wikipedia - version of the 11/18/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.