Aclarubicin
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | IV |
| ATC code | L01DB04 (WHO) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
57576-44-0 |
| PubChem (CID) | 42474 |
| ChemSpider |
1931 |
| UNII |
74KXF8I502 |
| KEGG |
D02756 |
| ChEBI |
CHEBI:74619 |
| ChEMBL |
CHEMBL502620 |
| Chemical and physical data | |
| Formula | C42H53NO15 |
| Molar mass | 811.86 g/mol |
| 3D model (Jmol) | Interactive image |
| Melting point | 151 to 153 °C (304 to 307 °F) (decomposes) |
| |
| |
| | |
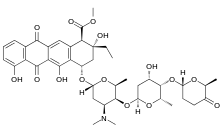
Aclarubicin (INN) or aclacinomycin A[1] is an anthracycline drug[2] that is used in the treatment of cancer. Soil bacteria Streptomyces galilaeus can produce aclarubicin. It can induce histone eviction from chromatin upon intercalation.[3][4]
References
- ↑ CID 451415 from PubChem
- ↑ Jensen PB, Jensen PS, Demant EJ, et al. (October 1991). "Antagonistic effect of aclarubicin on daunorubicin-induced cytotoxicity in human small cell lung cancer cells: relationship to DNA integrity and topoisomerase II". Cancer Res. 51 (19): 5093–9. PMID 1655244.
- ↑ Pang B, Qiao X, Janssen L, Velds A, Groothuis T, Kerkhoven R, Nieuwland M, Ovaa H, Rottenberg S, van Tellingen O, Janssen J, Huijgens P, Zwart W, Neefjes J (2013). "Drug-induced histone eviction from open chromatin contributes to the chemotherapeutic effects of doxorubicin". Nature Communications. 4: 1908. doi:10.1038/ncomms2921. PMID 23715267.
- ↑ Pang B, de Jong J, Qiao X, Wessels LF, Neefjes J (2015). "Chemical profiling of the genome with anti-cancer drugs defines target specificities". Nature Chemical Biology. 11 (7): 472. doi:10.1038/nchembio.1811. PMID 25961671.
This article is issued from Wikipedia - version of the 7/26/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.