Lycopene
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(6E,8E,10E,12E,14E,16E,18E,20E,22E,24E,26E)- 2,6,10,14,19,23,27,31-Octamethyldotriaconta-2,6,8,10,12,14,16,18,20,22,24,26,30-tridecaene | |
| Other names
ψ,ψ-Carotene | |
| Identifiers | |
| 502-65-8 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:15948 |
| ChEMBL | ChEMBL501174 |
| ChemSpider | 394156 |
| ECHA InfoCard | 100.007.227 |
| EC Number | 207-949-1 |
| E number | E160d (colours) |
| PubChem | 446925 |
| UNII | SB0N2N0WV6 |
| |
| |
| Properties | |
| C40H56 | |
| Molar mass | 536.89 g·mol−1 |
| Appearance | deep red solid |
| Density | 0.889 g/cm3 |
| Melting point | 172–173 °C (342–343 °F; 445–446 K) |
| Boiling point | 660.9 °C (1,221.6 °F; 934.0 K) at 760 mmHg[1] |
| insoluble | |
| Solubility | soluble in CS2, CHCl3, THF, ether, C6H14, vegetable oil insoluble in CH3OH, C2H5OH[1] |
| Solubility in hexane | 1 g/L (14 °C)[1] |
| Vapor pressure | 1.33·10−16 mmHg (25 °C)[1] |
| Hazards | |
| Main hazards | Combustible |
| Safety data sheet | See: data page |
| NFPA 704 | |
| Flash point | 350.7 °C (663.3 °F; 623.8 K) [1] |
| Supplementary data page | |
| Refractive index (n), Dielectric constant (εr), etc. | |
| Thermodynamic data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Lycopene from the neo-Latin lycopersicum, the tomato species, is a bright red carotene and carotenoid pigment and phytochemical found in tomatoes and other red fruits and vegetables, such as red carrots, watermelons, gac, and papayas, although not in strawberries, or cherries.[2] Although lycopene is chemically a carotene, it has no vitamin A activity.[3] Foods that are not red may also contain lycopene, such as asparagus and parsley.[2]
In plants, algae, and other photosynthetic organisms, lycopene is an important intermediate in the biosynthesis of many carotenoids, including beta carotene, which is responsible for yellow, orange, or red pigmentation, photosynthesis, and photo-protection. Like all carotenoids, lycopene is a polyunsaturated hydrocarbon, i.e. an unsubstituted alkene. Structurally, lycopene is a tetraterpene and assembled from eight isoprene units that are composed entirely of carbon and hydrogen. It is insoluble in water. Lycopene's eleven conjugated double bonds give its deep red color and its antioxidant activity. Owing to the strong color, lycopene is a useful food coloring (registered as E160d) and is approved for usage in the USA,[4] Australia and New Zealand (registered as 160d)[5] and the EU.[6]
Consumption by humans
Lycopene is not an essential nutrient for humans, but is commonly found in the diet mainly from dishes prepared from tomatoes.[2] When absorbed from the intestine, lycopene is transported in the blood by various lipoproteins and accumulates primarily in the blood, adipose tissue, skin, liver, and adrenal glands, but can be found in most tissues.
 |
.png) |
Structure and physical properties
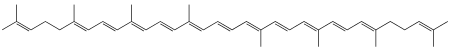
Lycopene is a symmetrical tetraterpene assembled from eight isoprene units. It is a member of the carotenoid family of compounds, and because it consists entirely of carbon and hydrogen, is also a carotene.[7] Isolation procedures for lycopene were first reported in 1910, and the structure of the molecule was determined by 1931. In its natural, all-trans form, the molecule is long and straight, constrained by its system of eleven conjugated double bonds. Each extension in this conjugated system reduces the energy required for electrons to transition to higher energy states, allowing the molecule to absorb visible light of progressively longer wavelengths. Lycopene absorbs all but the longest wavelengths of visible light, so it appears red.[8]
Plants and photosynthetic bacteria naturally produce all-trans lycopene, but a total of 72 geometric isomers of the molecule are sterically possible.[9] When exposed to light or heat, lycopene can undergo isomerization to any of a number of these cis-isomers, which have a bent rather than linear shape. Different isomers were shown to have different stabilities due to their molecular energy (highest stability: 5-cis ≥ all-trans ≥ 9-cis ≥ 13-cis > 15-cis > 7-cis > 11-cis: lowest).[10] In the human bloodstream, various cis-isomers constitute more than 60% of the total lycopene concentration, but the biological effects of individual isomers have not been investigated.[11]
Staining and removal
Lycopene, the pigment in tomato-containing sauces, turns plastic cookware orange and is insoluble in water. It can be dissolved only in organic solvents and oils. Because of its non-polarity, lycopene in food preparations will stain any sufficiently porous material, including most plastics. To remove this staining, the plastics can be soaked in a solution containing a small amount of household bleach.[12]
Role in photosynthesis

Carotenoids like lycopene are important pigments found in photosynthetic pigment-protein complexes in plants, photosynthetic bacteria, fungi, and algae. They are responsible for the bright colors of fruits and vegetables, perform various functions in photosynthesis, and protect photosynthetic organisms from excessive light damage. Lycopene is a key intermediate in the biosynthesis of many important carotenoids, such as beta-carotene, and xanthophylls.[13]
Biosynthesis
The unconditioned biosynthesis of lycopene in eukaryotic plants and in prokaryotic cyanobacteria is similar, as are the enzymes involved.[14] Synthesis begins with mevalonic acid, which is converted into dimethylallyl pyrophosphate. This is then condensed with three molecules of isopentenyl pyrophosphate (an isomer of dimethylallyl pyrophosphate), to give the twenty-carbon geranylgeranyl pyrophosphate. Two molecules of this product are then condensed in a tail-to-tail configuration to give the forty-carbon phytoene, the first committed step in carotenoid biosynthesis. Through several desaturation steps, phytoene is converted into lycopene. The two terminal isoprene groups of lycopene can be cyclized to produce beta-carotene, which can then be transformed into a wide variety of xanthophylls.[15]
Dietary sources
| Source | μg/g wet weight |
|---|---|
| Gac | 2,000–2,300 |
| Raw tomato | 8.8–42 |
| Tomato juice | 86–100 |
| Tomato sauce | 63–131 |
| Tomato ketchup | 124 |
| Watermelon | 23–72 |
| Pink grapefruit | 3.6–34 |
| Pink guava | 54 |
| Papaya | 20–53 |
| Rosehip puree | 7.8 |
| Apricot | < 0.1 |
Fruits and vegetables that are high in lycopene include autumn olive, gac, tomatoes, watermelon, pink grapefruit, pink guava, papaya, seabuckthorn, wolfberry (goji, a berry relative of tomato), and rosehip. Although gac (Momordica cochinchinensis Spreng) has the highest content of lycopene of any known fruit or vegetable, up to 70 times more than tomatoes for example,[17] due to gac's rarity outside its native region of southeast Asia, tomatoes and tomato-based sauces, juices, and ketchup account for more than 85% of the dietary intake of lycopene for most people.[18] The lycopene content of tomatoes depends on species and increases as the fruit ripens.[19]
Unlike other fruits and vegetables, where nutritional content such as vitamin C is diminished upon cooking, processing of tomatoes increases the concentration of bioavailable lycopene.[20] Lycopene in tomato paste is up to four times more bioavailable than in fresh tomatoes.[21]
While most green leafy vegetables and other sources of lycopene are low in fats and oils, lycopene is insoluble in water and is tightly bound to vegetable fiber. Processed tomato products such as pasteurized tomato juice, soup, sauce, and ketchup contain the highest concentrations of bioavailable lycopene from tomato-based sources.
Cooking and crushing tomatoes (as in the canning process) and serving in oil-rich dishes (such as spaghetti sauce or pizza) greatly increases assimilation from the digestive tract into the bloodstream. Lycopene is fat-soluble, so the oil is said to help absorption. Gac is a notable exception, containing high concentrations of lycopene and also saturated and unsaturated fatty acids.[22]
Lycopene may be obtained from vegetables and fruits such as the tomato, but another source of lycopene is the fungus Blakeslea trispora. Gac is a possible commercial source of lycopene for the purposes of extraction and purification, as its seed content of lycopene is high.[23]
The cis-lycopene from some varieties of tomato is more bioavailable.[24]
Note that there are some resources which make the mistaken assumption that all red fruits contain lycopene, when in fact many are pigmented by other chemicals. An example is the blood orange, which is colored by anthocyanins,[25] while other red colored oranges, such as the Cara cara navel, and other citrus fruit, such as pink grapefruit, are colored by lycopene.[2][26]
In addition, some foods that do not appear red also contain lycopene, e.g., asparagus, which contains approximately 30μg of lycopene per 100 gram serving[2] (0.3μg/g) and dried parsley and basil, which contain approximately 3.5-7 μg of lycopene per gram.[2]
Metabolism
| Tissue | nmol/g wet weight |
|---|---|
| Liver | 1.28–5.72 |
| Kidney | 0.15–0.62 |
| Adrenal | 1.9–21.6 |
| Testes | 4.34–21.4 |
| Ovary | 0.25–0.28 |
| Adipose | 0.2–1.3 |
| Lung | 0.22–0.57 |
| Colon | 0.31 |
| Breast | 0.78 |
| Skin | 0.42 |
After ingestion, lycopene is incorporated into lipid micelles in the small intestine. These micelles are formed from dietary fats and bile acids, and help to solubilize the hydrophobic lycopene and allow it to permeate the intestinal mucosal cells by a passive transport mechanism. Little is known about the liver metabolism of lycopene, but like other carotenoids, lycopene is incorporated into chylomicrons and released into the lymphatic system. In blood plasma, lycopene is eventually distributed into the very low and low density lipoprotein fractions.[28] Lycopene is mainly distributed to fatty tissues and organs such as the adrenal glands, liver, prostate and testes.
Adverse effects

Lycopene is non-toxic and is commonly found in the diet, but cases of excessive carotenoid intake have been reported. In a middle-aged woman who had prolonged and excessive consumption of tomato juice, her skin and liver were colored orange-yellow and she had elevated levels of lycopene in her blood. After three weeks on a lycopene-free diet her skin color returned to normal.[28] This discoloration of the skin is known as lycopenodermia[29] and is non-toxic.
There are also cases of intolerance or allergic reaction to dietary lycopene, which may cause diarrhea, nausea, stomach pain or cramps, gas, vomiting, and loss of appetite.[30]
Potential health effects
Lycopene from tomatoes has been tested in human studies for cardiovascular diseases and prostate cancer. These studies, however, did not attain sufficient scientific agreement to conclude an effect on any disease.[31] The FDA, in rejecting manufacturers' requests in 2005 to allow "qualified labeling" for lycopene and the reduction of various cancer risks, stated:
"...no studies provided information about whether lycopene intake may reduce the risk of any of the specific forms of cancer. Based on the above, FDA concludes that there is no credible evidence supporting a relationship between lycopene consumption, either as a food ingredient, a component of food, or as a dietary supplement, and any of these cancers."
A 2011 Cochrane review found insufficient evidence for any effect lycopene might have on prostate symptoms, PSA levels or prostate cancer.[32]
See also
References
- 1 2 3 4 5 "Lycopene". PubChem, US National Library of Medicine. 2016. Retrieved 13 October 2016.
- 1 2 3 4 5 6 "Foods highest in lycopene, Nutrition Data, USDA Nutrient Database, version SR-21". nutritiondata.com. Conde Nast. 2014. Retrieved 2014-08-19.
- ↑ Journal of the American College of Nutrition: Role of Antioxidant Lycopene in Cancer and Heart Disease
- ↑ 21 CFR 73.585
- ↑ Australia New Zealand Food Standards Code"Standard 1.2.4 - Labelling of ingredients". Retrieved 2011-10-27.
- ↑ UK Food Standards Agency: "Current EU approved additives and their E Numbers". Retrieved 2011-10-27.
- ↑ Grossman et al. (2004) p. 129
- ↑ Rao et al. (2007) p. 210
- ↑ 1054 isomers are theoretically possible, but only 72 are possible due to steric hindrance. IARC Handbook, (1998) p. 25
- ↑ Chasse et al. Journal of Molecular Structure: THEOCHEM, Volume 571, Number 1, 27 August 2001 , pp. 27-37(11)
- ↑ Lycopene: Its role in human health and disease, Rao 'et al.', AGROFood industry hi-tech, July/August 2003
- ↑ http://www.huffingtonpost.com/chris-barnes/how-to-clean-tomato-sauce_b_1521201.html
- ↑ NDSU Agriculture. "What Color is Your Food?". Retrieved 10 May 2012.
- ↑ Cunningham (2007) p. 533
- ↑ Armstrong (1996) p. 229
- ↑ Rao and Rao (2007) pp. 209–210
- ↑ USDA study on Cartenoid content of gac fruit
- ↑ Rao (2007) p.
- ↑ Khan et al. (2008) p. 495
- ↑ Perdomo F, Cabrera Fránquiz F, Cabrera J, Serra-Majem L (2012). "Influence of cooking procedure on the bioavailability of lycopene in tomatoes". Hospital Nutrition (Madrid). 27 (5): 1542–6. doi:10.3305/nh.2012.27.5.5908. PMID 23478703.
- ↑ Kamiloglu, S.; Demirci, M.; Selen, S.; Toydemir, G.; Boyacioglu, D.; Capanoglu, E. (2014). "Home processing of tomatoes (Solanum lycopersicum): Effects onin vitrobioaccessibility of total lycopene, phenolics, flavonoids, and antioxidant capacity". Journal of the Science of Food and Agriculture. 94 (11): 2225–33. doi:10.1002/jsfa.6546. PMID 24375495.
- ↑ Ishida BK, Turner C, Chapman MH, McKeon TA (January 2004). "Fatty acid and carotenoid composition of gac (Momordica cochinchinensis Spreng) fruit". Journal of Agricultural and Food Chemistry. 52 (2): 274–9. doi:10.1021/jf030616i. PMID 14733508.
- ↑ Aoki, H; Kieu, N. T.; Kuze, N; Tomisaka, K; Van Chuyen, N (2002). "Carotenoid pigments in GAC fruit (Momordica cochinchinensis SPRENG)". Bioscience, Biotechnology and Biochemistry. 66 (11): 2479–82. doi:10.1271/bbb.66.2479. PMID 12506992.
- ↑ "Unique Tomatoes Tops In Disease-Fighting Antioxidants". Medical News Today. March 5, 2007. Retrieved 2014-08-19.
- ↑ Hillebrand, S; Schwarz, M; Winterhalter, P (2004). "Characterization of anthocyanins and pyranoanthocyanins from blood orange Citrus sinensis (L.) Osbeck juice". Journal of Agricultural and Food Chemistry. 52 (24): 7331–8. doi:10.1021/jf0487957. PMID 15563216.
- ↑ Alquezar, B; Rodrigo, M. J.; Zacarías, L (2008). "Regulation of carotenoid biosynthesis during fruit maturation in the red-fleshed orange mutant Cara Cara". Phytochemistry. 69 (10): 1997–2007. doi:10.1016/j.phytochem.2008.04.020. PMID 18538806.
- ↑ Stahl (1996) p. 7
- 1 2 Stahl (1996) p. 6
- ↑ Institute of Medicine, Food and Nutrition Board. Beta-carotene and other carotenoids. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, D.C.: National Academy Press; 2000:325-400.
- ↑ "Lycopene". Mayo Clinic. October 1, 2011. Retrieved December 17, 2011.
- ↑ "Qualified Health Claims: Letter Regarding Tomatoes and Prostate Cancer (Lycopene Health Claim Coalition) (Docket No. 2004Q-0201)". US Food and Drug Administration. 8 November 2005.
- ↑ Ilic, D.; Forbes, KM.; Hassed, C. (2011). "Lycopene for the prevention of prostate cancer.". Cochrane Database Syst Rev (11): CD008007. doi:10.1002/14651858.CD008007.pub2. PMID 22071840.
External links
| Wikimedia Commons has media related to Lycopene. |
