Heritability of autism
The heritability of autism is the proportion of differences in expression of autism that can be explained by genetic variation; if the heritability of a condition is high, then the condition is considered to be primarily genetic. Autism has a strong genetic basis, although the genetics of autism is complex and it is unclear whether autism spectrum disorder (ASD) is explained more by multigene interactions or by rare mutations with major effects.[1]
Early studies of twins estimated the heritability of autism to be more than 90%--meaning that 90% of the differences between autistic and non-autistic individuals was due to genetics.[2] This may be an overestimate: new twin data and models with structural genetic variation are needed.[3] When only one identical twin is autistic, the other often has learning or social disabilities.[4] For adult siblings, the risk of having one or more features of the broader autism phenotype might be as high as 30%,[5] much higher than the risk in controls.[6]
Genetic linkage analysis has been inconclusive; many association analyses have had inadequate power.[3] For each autistic individual, mutations in more than one gene may be implicated. Mutations in different sets of genes may be involved in different autistic individuals. There may be significant interactions among mutations in several genes, or between the environment and mutated genes. By identifying genetic markers inherited with autism in family studies, numerous candidate genes have been located, most of which encode proteins involved in neural development and function.[7][8] However, for most of the candidate genes, the actual mutations that increase the risk for autism have not been identified. Typically, autism cannot be traced to a Mendelian (single-gene) mutation or to single chromosome abnormalities such as fragile X syndrome or 22q13 deletion syndrome.[9][10]
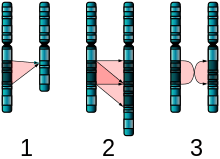
The large number of autistic individuals with unaffected family members may result from copy number variations (CNVs)—spontaneous alterations in the genetic material during meiosis that delete or duplicate genetic material.[12][13] Sporadic (non-inherited) cases have been examined to identify candidate genetic loci involved in autism. A substantial fraction of autism may be highly heritable but not inherited: that is, the mutation that causes the autism is not present in the parental genome.[11]
Although the fraction of autism traceable to a genetic cause may grow to 30–40% as the resolution of array CGH improves,[11] several results in this area have been described incautiously, possibly misleading the public into thinking that a large proportion of autism is caused by CNVs and is detectable via array CGH, or that detecting CNVs is tantamount to a genetic diagnosis.[14] The Autism Genome Project database contains genetic linkage and CNV data that connect autism to genetic loci and suggest that every human chromosome may be involved.[15] It may be that using autism-related subphenotypes instead of the diagnosis of autism per se may be more useful in identifying susceptible loci.[16]
Twin studies
Twin studies are a helpful tool in determining the heritability of disorders and human traits in general. They involve determining concordance of characteristics between identical (monozygotic or MZ) twins and between fraternal (dizygotic or DZ) twins. Possible problems of twin studies are: (1) errors in diagnosis of monozygocity, and (2) the assumption that social environment sharing by DZ twins is equivalent to that of MZ twins.
A condition that is environmentally caused without genetic involvement would yield a concordance for MZ twins equal to the concordance found for DZ twins. In contrast, a condition that is completely genetic in origin would theoretically yield a concordance of 100% for MZ pairs and usually much less for DZ pairs depending on factors such as the number of genes involved and assortative mating.
An example of a condition that appears to have very little if any genetic influence is irritable bowel syndrome (IBS), with a concordance of 28% vs. 27% for MZ and DZ pairs respectively.[17] An example of a human characteristics that is extremely heritable is eye color, with a concordance of 98% for MZ pairs and 7–49% for DZ pairs depending on age.[18]
Identical twin studies put autism's heritability in a range between 36% and 95.7%, with concordance for a broader phenotype usually found at the higher end of the range.[19] Autism concordance in siblings and fraternal twins is anywhere between 0 and 23.5%. This is more likely 2–4% for classic autism and 10–20% for a broader spectrum. Assuming a general-population prevalence of 0.1%, the risk of classic autism in siblings is 20- to 40-fold that of the general population.
Notable twin studies have attempted to shed light on the heritability of autism.
A small scale study in 1977 was the first of its kind to look into the heritability of autism. It involved 10 DZ and 11 MZ pairs in which at least one twin in each pair showed infantile autism. It found a concordance of 36% in MZ twins compared to 0% for DZ twins. Concordance of "cognitive abnormalities" was 82% in MZ pairs and 10% for DZ pairs. In 12 of the 17 pairs discordant for autism, a biological hazard was believed to be associated with the condition.[20]
A 1979 case report discussed a pair of identical twins concordant for autism. The twins developed similarly until the age of 4, when one of them spontaneously improved. The other twin, who had suffered infrequent seizures, remained autistic. The report noted that genetic factors were not "all important" in the development of the twins.[21]
In 1985, a study of twins enrolled with the UCLA Registry for Genetic Studies found a concordance of 95.7% for autism in 23 pairs of MZ twins, and 23.5% for 17 DZ twins.[22]
In a 1989 study, Nordic countries were screened for cases of autism. Eleven pairs of MZ twins and 10 of DZ twins were examined. Concordance of autism was found to be 91% in MZ and 0% in DZ pairs. The concordances for "cognitive disorder" were 91% and 30% respectively. In most of the pairs discordant for autism, the autistic twin had more perinatal stress.[23]
A British twin sample was reexamined in 1995 and a 60% concordance was found for autism in MZ twins vs. 0% concordance for DZ. It also found 92% concordance for a broader spectrum in MZ vs. 10% for DZ. The study concluded that "obstetric hazards usually appear to be consequences of genetically influenced abnormal development, rather than independent aetiological factors."[24]
A 1999 study looked at social cognitive skills in general-population children and adolescents. It found "poorer social cognition in males", and a heritability of 0.68 with higher genetic influence in younger twins.[25]
In 2000, a study looked at reciprocal social behavior in general-population identical twins. It found a concordance of 73% for MZ, i.e. "highly heritable", and 37% for DZ pairs.[26]
A 2004 study looked at 16 MZ twins and found a concordance of 43.75% for "strictly defined autism". Neuroanatomical differences (discordant cerebellar white and grey matter volumes) between discordant twins were found. The abstract notes that in previous studies 75% of the non-autistic twins displayed the broader phenotype.[27]
Another 2004 study examined whether the characteristic symptoms of autism (impaired social interaction, communication deficits, and repetitive behaviors) show decreased variance of symptoms among monozygotic twins compared to siblings in a sample of 16 families. The study demonstrated significant aggregation of symptoms in twins. It also concluded that "the levels of clinical features seen in autism may be a result of mainly independent genetic traits."[28]
An English twin study in 2006 found high heritability for autistic traits in a large group of 3,400 pairs of twins.[29]
One critic of the pre-2006 twin studies said that they were too small and their results can be plausibly explained on non-genetic grounds.[30]
Sibling studies
A study of 99 autistic probands which found a 2.9% concordance for autism in siblings, and between 12.4% and 20.4% concordance for a "lesser variant" of autism.[6]
A study of 31 siblings of autistic children, 32 siblings of children with developmental delay, and 32 controls. It found that the siblings of autistic children, as a group, "showed superior spatial and verbal span, but a greater than expected number performed poorly on the set-shifting, planning, and verbal fluency tasks."[31]
A 2005 Danish study looked at "data from the Danish Psychiatric Central Register and the Danish Civil Registration System to study some risk factors of autism, including place of birth, parental place of birth, parental age, family history of psychiatric disorders, and paternal identity." It found an overall prevalence rate of roughly 0.08%. Prevalence of autism in siblings of autistic children was found to be 1.76%. Prevalence of autism among siblings of children with Asperger syndrome or PDD was found to be 1.04%. The risk was twice as high if the mother had been diagnosed with a psychiatric disorder. The study also found that "the risk of autism was associated with increasing degree of urbanisation of the child's place of birth and with increasing paternal, but not maternal, age."[32]
A study in 2007 looked at a database containing pedigrees of 86 families with two or more autistic children and found that 42 of the third-born male children showed autistic symptoms, suggesting that parents had a 50% chance of passing on a mutation to their offspring. The mathematical models suggest that about 50% of autistic cases are caused by spontaneous mutations. The simplest model was to divide parents into two risk classes depending on whether the parent carries a pre-existing mutation that causes autism; it suggested that about a quarter of autistic children have inherited a copy number variation from their parents.[33]
Other family studies
A 1994 study looked at the personalities of parents of autistic children, using parents of children with Down's syndrome as controls. Using standardized tests it was found that parents of autistic children were "more aloof, untactful and unresponsive" compared to parents whose children did not have autism.[34]
A 1997 study found higher rates of social and communication deficits and stereotyped behaviors in families with multiple-incidence autism.[35]
Autism was found to occur more often in families of physicists, engineers and scientists. 12.5% of the fathers and 21.2% of the grandfathers (both paternal and maternal) of children with autism were engineers, compared to 5% of the fathers and 2.5% of the grandfathers of children with other syndromes.[36] Other studies have yielded similar results.[37][38] Findings of this nature have led to the coinage of the term "geek syndrome".[39]
A 2001 study of brothers and parents of autistic boys looked into the phenotype in terms of one current cognitive theory of autism. The study raised the possibility that the broader autism phenotype may include a "cognitive style" (weak central coherence) that can confer information-processing advantages.[40]
A study in 2005 showed a positive correlation between repetitive behaviors in autistic individuals and obsessive-compulsive behaviors in parents.[41] Another 2005 study focused on sub-threshold autistic traits in the general population. It found that correlation for social impairment or competence between parents and their children and between spouses is about 0.4.[42]
A 2005 report examined the family psychiatric history of 58 subjects with Asperger syndrome (AS) diagnosed according to DSM-IV criteria. Three (5%) had first-degree relatives with AS. Nine (19%) had a family history of schizophrenia. Thirty five (60%) had a family history of depression. Out of 64 siblings, 4 (6.25%) were diagnosed with AS.[43]
Twinning risk
It has been suggested that the twinning process itself is a risk factor in the development of autism, presumably due to perinatal factors.[44] However, three large-scale epidemiological studies have refuted this idea.[2][45]
Proposed models
Twin and family studies show that autism is a highly heritable condition, but they have left many questions for researchers, most notably
- Why is fraternal twin concordance so low considering that identical twin concordance is high?
- Why are parents of autistic children typically non-autistic?
- Which factors could be involved in the failure to find a 100% concordance in identical twins?
- Is profound intellectual disability a characteristic of the genotype or something totally independent?
Clues to the first two questions come from studies that have shown that at least 30% of individuals with autism have spontaneous de novo mutations that occurred in the father's sperm or mother's egg and disrupt genes important for brain development, these spontaneous mutations likely cause autism in families where there is no family history.[46] The concordance between identical twins isn't quite 100% for two reasons, because these mutations have variable 'expressivity' and their effects manifest differently due to chance effects, epigenetic, and environmental factors. Also spontaneous mutations can potentially occur specifically in one twin and not the other after conception.[47] The likelihood of developing intellectual disability is dependent on the importance the effect the gene or mutation has on brain development, and also the genetic and environmental background upon which a mutation occurs.[48] The recurrence of the same mutations in multiple individuals affected by autism has led Brandler and Sebat to suggest that the spectrum of autism is breaking up into quanta of many different genetic disorders.[48]
Single genes
The most parsimonious explanation for cases of autism where a single child is affected and there is no family history or affected siblings is that a single spontaneous mutation that impacts one or multiple genes is a significant contributing factor.[48][49] Tens of individual genes or mutations have been definitively identified and are cataloged by the Simons Foundation Autism Research Initiative.[50][51] Examples of autism that has arisen from a rare or de novo mutation in a single-gene or locus include the neurodevelopmental disorders fragile X syndrome, 22q13 deletion syndrome, and 16p11.2 deletion syndrome.[52]
These mutations themselves are characterized by considerable variability in clinical outcome and typically only a subset of mutation carriers meet criteria for autism. For example, carriers of the 16p11.2 deletion have a mean IQ 32 points lower than their first-degree relatives that do not carry the deletion, however only 20% are below the threshold IQ of 70 for intellectual disability, and only 20% have autism.[53][54] Around 85% have a neurobehavioral diagnosis, including autism, ADHD, anxiety disorders, mood disorders, gross motor delay, and epilepsy, while 15% have no diagnosis.[54] Alongside these neurobehavioral phenotypes, the 16p11.2 deletions / duplications have been associated with macrocephaly / microcephaly, body weight regulation, and the duplication in particular is associated with schizophrenia.[53][55][56] Controls that carry mutations associated with autism or schizophrenia typically present with intermediate cognitive phenotypes or fecundity compared to neurodevelopmental cases and population controls.[57] Therefore, a single mutation can have multiple different effects depending on other genetic and environmental factors.
Multigene interactions
In this model, autism often arises from a combination of common, functional variants of genes. Each gene contributes a relatively small effect in increasing the risk of autism. In this model, no single gene directly regulates any core symptom of autism such as social behavior. Instead, each gene encodes a protein that disrupts a cellular process, and the combination of these disruptions, possibly together with environmental influences,[58] affect key developmental processes such as synapse formation. For example, one model is that many mutations affect MET and other receptor tyrosine kinases, which in turn converge on disruption of ERK and PI3K signaling.[52]
Two family types
In this model most families fall into two types: in the majority, sons have a low risk of autism, but in a small minority their risk is near 50%. In the low-risk families, sporadic autism is mainly caused by spontaneous mutation with poor penetrance in daughters and high penetrance in sons. The high-risk families come from (mostly female) children who carry a new causative mutation but are unaffected and transmit the dominant mutation to grandchildren.[59]
Epigenetic
Several epigenetic models of autism have been proposed.[60] These are suggested by the occurrence of autism in individuals with fragile X syndrome, which arises from epigenetic mutations, and with Rett syndrome, which involves epigenetic regulatory factors. An epigenetic model would help explain why standard genetic screening strategies have so much difficulty with autism.[61]
Genomic imprinting
Genomic imprinting models have been proposed; one of their strengths is explaining the high male-to-female ratio in ASD.[62] One hypothesis is that autism is in some sense diametrically opposite to schizophrenia and other psychotic-spectrum conditions, that alterations of genomic imprinting help to mediate the development of these two sets of conditions, and that ASD involves increased effects of paternally expressed genes, which regulate overgrowth in the brain, whereas schizophrenia involves maternally expressed genes and undergrowth.[63]
Environmental interactions
Though autism's genetic factors explain most of autism risk, they do not explain all of it. A common hypothesis is that autism is caused by the interaction of a genetic predisposition and an early environmental insult.[64] Several theories based on environmental factors have been proposed to address the remaining risk. Some of these theories focus on prenatal environmental factors, such as agents that cause birth defects; others focus on the environment after birth, such as children's diets. All known teratogens (agents that cause birth defects) related to the risk of autism appear to act during the first eight weeks from conception, strong evidence that autism arises very early in development.[65] Although evidence for other environmental causes is anecdotal and has not been confirmed by reliable studies,[66] extensive searches are underway.[67]
Candidate gene loci
Known genetic syndromes, mutations, and metabolic diseases account for up to 20% of autism cases.[68] A number of alleles have been shown to have strong linkage to the autism phenotype. In many cases the findings are inconclusive, with some studies showing no linkage. Alleles linked so far strongly support the assertion that there is a large number of genotypes that are manifested as the autism phenotype. At least some of the alleles associated with autism are fairly prevalent in the general population, which indicates they are not rare pathogenic mutations. This also presents some challenges in identifying all the rare allele combinations involved in the etiology of autism.
A 2008 study compared genes linked with autism to those of other neurological diseases, and found that more than half of known autism genes are implicated in other disorders, suggesting that the other disorders may share molecular mechanisms with autism.[69]
Primary
| Gene | OMIM/# | Locus | Description |
| CDH9, CDH10 | 5p14.1 | A 2009 pair of genome-wide association studies found an association between autism and six single-nucleotide polymorphisms in an intergenic region between CDH10 (cadherin 10) and CDH9 (cadherin 9). These genes encode neuronal cell-adhesion molecules, implicating these molecules in the mechanism of autism.[70] | |
| CDH8 | 16q21 | A family based study identified a deletion of CDH8 that was transmitted to three out of three affected children and zero out of four unaffected siblings.[71] Further evidence for the role of CDH8 comes from a spontaneous 1.52 megabase inversion that disrupts the gene in an affected child.[72] | |
| MAPK3 | 16p11.2 | A 2008 study observed a de novo deletion of 593 kb on this chromosome in about 1% of persons with autism, and similarly for the reciprocal duplication of the region.[73] Another 2008 study also found duplications and deletions associated with ASD at this locus.[74] This gene encodes ERK1, one of the extracellular signal regulated kinase subfamily of mitogen-activated protein kinases which are central elements of an intracellular signaling pathways that transmits signals from cell surfaces to interiors. 1% of autistic children have been found to have either a loss or duplication in a region of chromosome 16 that encompasses the gene for ERK1. A similar disturbance in this pathway is also found in neuro-cardio-facial-cutaneous syndromes (NCFC), which are characterized by cranio-facial development disturbances that also can be found in some cases of autism.[75] | |
| SERT (SLC6A4) | 17q11.2 | This gene locus has been associated with rigid-compulsive behaviors. Notably, it has also been associated with depression but only as a result of social adversity, although other studies have found no link.[76] Significant linkage in families with only affected males has been shown.[77][78] Researchers have also suggested that the gene contributes to hyperserotonemia.[79] However, a 2008 meta-analysis of family- and population-based studies found no significant overall association between autism and either the promoter insertion/deletion (5-HTTLPR) or the intron 2 VNTR (STin2 VNTR) polymorphisms.[80] | |
| CACNA1G | 17q21.33 | Markers within an interval containing this gene are associated with ASD at a locally significant level. The region likely harbors a combination of multiple rare and common alleles that contribute to genetic risk for ASD.[81] | |
| GABRB3, GABRA4 | multiple | GABA is the primary inhibitory neurotransmitter of the human brain. Ma et al. (2005) concluded that GABRA4 is involved in the etiology of autism, and that it potentially increases autism risk through interaction with GABRB1.[82] The GABRB3 gene has been associated with savant skills.[83] The GABRB3 gene deficient mouse has been proposed as a model of ASD.[84] | |
| Engrailed 2 (EN2) | 7q36.2 | Engrailed 2 is believed to be associated with cerebellar development. Benayed et al.. (2005) estimate that this gene contributes to as many as 40% of ASD cases, about twice the prevalence of the general population.[85] But at least one study has found no association.[86] | |
| ? | 3q25-27 | A number of studies have shown a significant linkage of autism and Asperger syndrome with this locus.[87][88] The most prominent markers are in the vicinity of D3S3715 and D3S3037.[89] | |
| Reelin | 7q21-q36 | In adults, Reelin glycoprotein is believed to be involved in memory formation, neurotransmission, and synaptic plasticity. A number of studies have shown an association between the REELIN gene and autism,[90][91] but a couple of studies were unable to duplicate linkage findings.[92] | |
| SLC25A12 | 2q31 | This gene encodes the mitochondrial aspartate/glutamate carrier (AGC1). It has been found to have a significant linkage to autism in some studies,[93][94][95] but linkage was not replicated in others,[96] and a 2007 study found no compelling evidence of an association of any mitochondrial haplogroup in autism.[97] | |
| HOXA1 and HOXB1 | multiple | A link has been found between HOX genes and the development of the embryonic brain stem. In particular, two genes, HOXA1 and HOXB1, in transgenic 'knockout' mice, engineered so that these genes were absent from the genomes of the mice in question, exhibited very specific brain stem developmental differences from the norm, which were directly comparable to the brain stem differences discovered in a human brain stem originating from a diagnosed autistic patient.[98]
Conciatori et al.. (2004) found an association of HOXA1 with increased head circumference.[99] A number of studies have found no association with autism.[100][101][102] The possibility remains that single allelic variants of the HOXA1 gene are insufficient alone to trigger the developmental events in the embryo now associated with autistic spectrum conditions. Tischfield et al.. published a paper which suggests that because HOXA1 is implicated in a wide range of developmental mechanisms, a model involving multiple allelic variants of HOXA1 in particular may provide useful insights into the heritability mechanisms involved.[103] Additionally, Ingram et al.. alighted upon additional possibilities in this arena.[104] Transgenic mouse studies indicate that there is redundancy spread across HOX genes that complicate the issue, and that complex interactions between these genes could play a role in determining whether or not a person inheriting the requisite combinations manifests an autistic spectrum condition[105]—transgenic mice with mutations in both HOXA1 and HOXB1 exhibit far more profound developmental anomalies than those in which only one of the genes differs from the conserved 'norm'. In Rodier's original work, teratogens are considered to play a part in addition, and that the possibility remains open for a range of teratogens to interact with the mechanisms controlled by these genes unfavourably (this has already been demonstrated using valproic acid, a known teratogen, in the mouse model).[106] | |
| PRKCB1 | 16p11.2 | Philippi et al. (2005) found a strong association between this gene and autism. This is a recent finding that needs to be replicated.[107] | |
| MECP2 | 300496, AUTSX3 | Mutations in this gene can give rise to autism spectrum disorders and related postnatal neurodevelopmental disorders.[108] | |
| UBE3A | 15q11.2–q13 | The maternally expressed imprinted gene UBE3A has been associated with Angelman syndrome. MeCP2 deficiency results in reduced expression of UBE3A in some studies.[109] | |
| Shank3 (ProSAP2) | 22q13 | The gene called SHANK3 (also designated ProSAP2) regulates the structural organization of neurotransmitter receptors in post-synaptic dendritic spines making it a key element in chemical binding crucial to nerve cell communication.[110] SHANK3 is also a binding partner of chromosome 22q13 (i.e. a specific section of Chromosome 22) and neuroligin proteins; deletions and mutations of SHANK3, 22q13 (i.e. a specific section of Chromosome 22) and genes encoding neuroligins have been found in some people with autism spectrum disorders.[111]
Mutations in the SHANK3 gene have been strongly associated with the autism spectrum disorders. If the SHANK3 gene is not adequately passed to a child from the parent (haploinsufficiency) there will possibly be significant neurological changes that are associated with yet another gene, 22q13, which interacts with SHANK3. Alteration or deletion of either will effect changes in the other.[111] A deletion of a single copy of a gene on chromosome 22q13 has been correlated with global developmental delay, severely delayed speech or social communication disorders and moderate to profound delay of cognitive abilities. Behavior is described as "autistic-like" and includes high tolerance to pain and habitual chewing or mouthing[111] (see also 22q13 deletion syndrome). This appears to be connected to the fact that signal transmission between nerve cells is altered with the absence of 22q13. SHANK3 proteins also interact with neuroligins at the synapses of the brain further complicating the widespread effects of changes at the genetic level and beyond.[112] | |
| NLGN3 | 300425, AUTSX1 | Xq13 | Neuroligin is a cell surface protein (homologous to acetylcholinesterase and other esterases) that binds to synaptic membranes.[113] Neuroligins organize postsynaptic membranes that function to transmit nerve cell messages (excitatory) and stop those transmissions (inhibitory);[114] In this way, neuroligins help to ensure signal transitions between nerve cells. Neuroligins also regulate the maturation of synapses and ensure there are sufficient receptor proteins on the synaptic membrane.
Mice with a neuroligin-3 mutation exhibit poor social skills but increased intelligence.[115] Though not present in all individuals with autism, these mutations hold potential to illustrate some of the genetic components of spectrum disorders.[112] However, a 2008 study found no evidence for involvement of neuroligin-3 and neuroligin-4x with high-functioning ASD.[116] |
| MET | 7q31 | The MET gene (MET receptor tyrosine kinase gene) linked to brain development, regulation of the immune system, and repair of the gastrointestinal system, has been linked to autism. This MET gene codes for a protein that relays signals that turn on a cell's internal machinery. Impairing the receptor's signaling interferes with neuron migration and disrupts neuronal growth in the cerebral cortex and similarly shrinks the cerebellum—abnormalities also seen in autism.[117]
It is also known to play a key role in both normal and abnormal development, such as cancer metastases. A mutation of the gene, rendering it less active, has been found to be common amongst children with autism.[117] Mutation in the MET gene demonstrably raises risk of autism by 2.27 times.[118] | |
| neurexin 1 | 2q32 | In February 2007, researchers in the Autism Genome Project (an international research team composed of 137 scientists in 50 institutions) reported possible implications in aberrations of a brain-development gene called neurexin 1 as a cause of some cases of autism.[15] Linkage analysis was performed on DNA from 1,181 families in what was the largest-scale genome scan conducted in autism research at the time.
The objective of the study was to locate specific brain cells involved in autism to find regions in the genome linked to autism susceptibility genes. The focus of the research was copy number variations (CNVs), extra or missing parts of genes. Each person does not actually have just an exact copy of genes from each parent. Each person also has occasional multiple copies of one or more genes or some genes are missing altogether. The research team attempted to locate CNVs when they scanned the DNA. Neurexin 1 is one of the genes that may be involved in communication between nerve cells (neurons). Neurexin 1 and other genes like it are very important in determining how the brain is connected from cell to cell, and in the chemical transmission of information between nerve cells. These genes are particularly active very early in brain development, either in utero or in the first months or couple of years of life. In some families their autistic child had only one copy of the neurexin 1 gene. Besides locating another possible genetic influence (the findings were statistically insignificant), the research also reinforced the theory that autism involves many forms of genetic variations. A 2008 study implicated the neurexin 1 gene in two independent subjects with ASD, and suggested that subtle changes to the gene might contribute to susceptibility to ASD.[119] A Neurexin 1 deletion has been observed occurring spontaneously in an unaffected mother and was passed on to an affected child, suggesting that the mutation has incomplete penetrance.[72] | |
| CNTNAP2 | 7q35-q36 | Multiple 2008 studies have identified a series of functional variants in the CNTNAP2 gene, a member of the neurexin superfamily, that implicate it as contributing to autism.[58][120][121][122] | |
| FOXP2 | 7q31 | The FOXP2 gene is of interest because it is known to be associated with developmental language and speech deficits.[123][124] A 2008 study found that FOXP2 binds to and down-regulates CNTNAP2, and that the FOXP2-CNTNAP2 pathway links distinct syndromes involving disrupted language.[125] | |
| GSTP1 | 11q13 | A 2007 study suggested that the GSTP1*A haplotype of the glutathione S-transferase P1 gene (GSTP1) acts in the mother during pregnancy and increases the likelihood of autism in the child.[126] | |
| PRL, PRLR, OXTR | multiple | A 2014 meta-analysis found significant associations between autism and several single-nucleotide polymorphisms in the OXTR gene.[127] |
Others
There is a large number of other candidate loci which either should be looked at or have been shown to be promising. Several genome-wide scans have been performed identifying markers across many chromosomes.[128][129][130]
A few examples of loci that have been studied are the 17q21 region,[131][132] the 3p24-26 locus,[128] PTEN,[133] 15q11.2–q13[109] and deletion in the 22q11.2 area.[134]
Homozygosity mapping in pedigrees with shared ancestry and autism incidence has recently implicated the following candidate genes: PCDH10, DIA1 (formerly known as C3ORF58), NHE9, CNTN3, SCN7A, and RNF8. Several of these genes appeared to be targets of MEF2,[135][136] one of the transcription factors known to be regulated by neuronal activity[137] and that itself has also recently been implicated as an autism-related disorder candidate gene.[138]
References
- ↑ Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9(5):341–55. doi:10.1038/nrg2346. PMID 18414403.
- 1 2 Freitag CM. The genetics of autistic disorders and its clinical relevance: a review of the literature. Mol Psychiatry. 2007;12(1):2–22. doi:10.1038/sj.mp.4001896. PMID 17033636.
- 1 2 Sykes NH, Lamb JA. Autism: the quest for the genes. Expert Rev Mol Med. 2007;9(24):1–15. doi:10.1017/S1462399407000452. PMID 17764594.
- ↑ Le Couteur A., Bailey A., Goode S., Pickles A., Robertson S., Gottesman I., Rutter M.. A broader phenotype of autism: the clinical spectrum in twins. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1996;37(7):785–801. doi:10.1111/j.1469-7610.1996.tb01475.x. PMID 8923222.
- ↑ Folstein SE, Rosen-Sheidley B. Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet. 2001;2(12):943–55. doi:10.1038/35103559. PMID 11733747.
- 1 2 Bolton P, Macdonald H, Pickles A et al. A case-control family history study of autism. J Child Psychol Psychiatry. 1994;35(5):877–900. doi:10.1111/j.1469-7610.1994.tb02300.x. PMID 7962246.
- ↑ Persico AM, Bourgeron T. Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends Neurosci. 2006;29(7):349–58. doi:10.1016/j.tins.2006.05.010. PMID 16808981.
- ↑ Yang MS, Gill M. A review of gene linkage, association and expression studies in autism and an assessment of convergent evidence. Int J Dev Neurosci. 2007;25(2):69–85. doi:10.1016/j.ijdevneu.2006.12.002. PMID 17236739.
- ↑ Cohen D, Pichard N, Tordjman S et al. Specific genetic disorders and autism: clinical contribution towards their identification. J Autism Dev Disord. 2005;35(1):103–16. doi:10.1007/s10803-004-1038-2. PMID 15796126.
- ↑ Müller RA. The study of autism as a distributed disorder. Ment Retard Dev Disabil Res Rev. 2007;13(1):85–95. doi:10.1002/mrdd.20141. PMID 17326118.
- 1 2 3 Beaudet AL. Autism: highly heritable but not inherited. Nat Med. 2007;13(5):534–6. doi:10.1038/nm0507-534. PMID 17479094.
- ↑ Cook EH, Scherer SW. Copy-number variations associated with neuropsychiatric conditions. Nature. 2008;455(7215):919–23. doi:10.1038/nature07458. PMID 18923514.
- ↑ Gai X, et al. Rare structural variation of synapse and neurotransmission genes in autism. Mol Psychiatry. 2011;17(4):402–11. doi:10.1038/mp.2011.10. PMID 21358714.
- ↑ Tabor HK, Cho MK. Ethical implications of array comparative genomic hybridization in complex phenotypes: points to consider in research. Genet Med. 2007;9(9):626–31. doi:10.1097/GIM.0b013e3181485688. PMID 17873651.
- 1 2 Autism Genome Project Consortium. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39(3):319–28. doi:10.1038/ng1985. PMID 17322880. Lay summary: Yale, 2007-02-18. Corrigendum. Nat Genet. 2007;39(10):1285. doi:10.1038/ng1007-1285a.
- ↑ Liu XQ, Paterson AD, Szatmari P; The Autism Genome Project Consortium. Genome-wide linkage analyses of quantitative and categorical autism subphenotypes. Biol Psychiatry. 2008;64(7):561–70. doi:10.1016/j.biopsych.2008.05.023. PMID 18632090.
- ↑ Mohammed I, Cherkas LF, Riley SA, Spector TD, Trudgill NJ. Genetic influences in irritable bowel syndrome: a twin study. Am J Gastroenterol. 2005;100(6):1340–4. doi:10.1111/j.1572-0241.2005.41700.x. PMID 15929767.
- ↑ Bito LZ, Matheny A, Cruickshanks KJ, Nondahl DM, Carino OB. Eye color changes past early childhood. The Louisville Twin Study. Arch Ophthalmol. 1997;115(5):659–63. doi:10.1001/archopht.1997.01100150661017. PMID 9152135.
- ↑ Twin studies (concordance in brackets):
- (0.8–1) Ciaranello, Roland D. M.D The Neurobiology of Infantile Autism
- (0.8) Kallen, Ronald J. M.D CDC Reports a higher than expected prevalence of autism in Brick Township
- (0.91–0.93) Dawson, Geraldine Ph. D Written testimony Public Health Subcommittee, United States Senate
- (0.9) Lang, Leslie H.Study points to chromosome site of autism gene
- (0.6–0.92) Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113(5):e472–86. doi:10.1542/peds.113.5.e472. PMID 15121991.
- (0.6–0.8) Kurita H. [Current status of autism studies]. Seishin shinkeigaku zasshi = Psychiatria et neurologia Japonica. 2001;103(1):64–75. Japanese. PMID 11383012.
- ↑ Folstein S, Rutter M. Infantile autism: a genetic study of 21 twin pairs. Journal of child psychology and psychiatry, and allied disciplines. 1977;18(4):297–321. doi:10.1111/j.1469-7610.1977.tb00443.x. PMID 562353.
- ↑ Wessels WH, Pompe van Meerdervoort M. Monozygotic twins with early infantile autism. A case report. S Afr Med J. 1979;55(23):955–7. PMID 572995.
- ↑ Ritvo ER, Freeman BJ, Mason-Brothers A, Mo A, Ritvo AM. Concordance for the syndrome of autism in 40 pairs of afflicted twins. The American Journal of Psychiatry. 1985;142(1):74–7. doi:10.1176/ajp.142.1.74. PMID 4038442.
- ↑ Steffenburg S, Gillberg C, Hellgren L, et al. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. Journal of child psychology and psychiatry, and allied disciplines. 1989;30(3):405–16. doi:10.1111/j.1469-7610.1989.tb00254.x. PMID 2745591.
- ↑ Bailey A, Le Couteur A, Gottesman I, et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychological Medicine. 1995;25(1):63–77. doi:10.1017/S0033291700028099. PMID 7792363.
- ↑ Scourfield J, Martin N, Lewis G, McGuffin P. Heritability of social cognitive skills in children and adolescents. The British Journal of Psychiatry. 1999;175(6):559–64. doi:10.1192/bjp.175.6.559. PMID 10789354.
- ↑ Constantino JN, Todd RD. Genetic structure of reciprocal social behavior. The American Journal of Psychiatry. 2000;157(12):2043–5. doi:10.1176/appi.ajp.157.12.2043. PMID 11097975.
- ↑ Kates WR, Burnette CP, Eliez S, et al. Neuroanatomic variation in monozygotic twin pairs discordant for the narrow phenotype for autism. The American Journal of Psychiatry. 2004;161(3):539–46. doi:10.1176/appi.ajp.161.3.539. PMID 14992981.
- ↑ Kolevzon A, Smith CJ, Schmeidler J, Buxbaum JD, Silverman JM. Familial symptom domains in monozygotic siblings with autism. Am J Med Genet B Neuropsychiatr Genet. 2004;129(1):76–81. doi:10.1002/ajmg.b.30011. PMID 15274045.
- ↑ Ronald A, Happé F, Bolton P, et al. Genetic heterogeneity between the three components of the autism spectrum: a twin study. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(6):691–9. doi:10.1097/01.chi.0000215325.13058.9d. PMID 16721319.
- ↑ Joseph J. The Missing Gene: Psychiatry, Heredity, and the Fruitless Search for Genes. Algora; 2006 [Retrieved 2007-07-25]. ISBN 0-87586-410-4. Autism and genetics: much ado about very little.
- ↑ Hughes C, Plumet MH, Leboyer M. Towards a cognitive phenotype for autism: increased prevalence of executive dysfunction and superior spatial span amongst siblings of children with autism. Journal of child psychology and psychiatry, and allied disciplines. 1999;40(5):705–18. doi:10.1111/1469-7610.00487. PMID 10433405.
- ↑ Lauritsen MB, Pedersen CB, Mortensen PB. Effects of familial risk factors and place of birth on the risk of autism: a nationwide register-based study. Journal of child psychology and psychiatry, and allied disciplines. 2005;46(9):963–71. doi:10.1111/j.1469-7610.2004.00391.x. PMID 16108999.
- ↑ Zhao X, Leotta A, Kustanovich V, et al. A unified genetic theory for sporadic and inherited autism. Proc Natl Acad Sci USA. 2007;104(31):12831–6. doi:10.1073/pnas.0705803104. PMID 17652511. PMC 1933261. Lay summary: CSHL, 2007-07-23.
- ↑ Piven J, Wzorek M, Landa R, et al. Personality characteristics of the parents of autistic individuals. Psychological Medicine. 1994;24(3):783–95. doi:10.1017/S0033291700027938. PMID 7991760.
- ↑ Piven J, Palmer P, Jacobi D, Childress D, Arndt S. Broader autism phenotype: evidence from a family history study of multiple-incidence autism families. The American Journal of Psychiatry. 1997;154(2):185–90. doi:10.1176/ajp.154.2.185. PMID 9016266.
- ↑ Baron-Cohen S, Bolton P, Wheelwright S et al.. Autism occurs more often in families of physicists, engineers, and mathematicians". (PDF) [PDF]. Autism. 1998;2:296–301. doi:10.1177/1362361398023008.
- ↑ Baron-Cohen S, Wheelwright S, Stott C et al.. Is there a link between engineering and autism?" (PDF) [PDF]. Autism. 1997;1:153–163.
- ↑ Wheelwright S, Baron-Cohen S. The link between autism and skills such as engineering, maths, physics and computing: a reply to Jarrold and Routh. Autism. 2001;5(2):223–7. doi:10.1177/1362361301005002010. PMID 11706868.Online.
- ↑ Silberman, Steve. The Geek Syndrome. Wired Magazine (December 2001). Retrieved on December 10, 2006.
- ↑ Happé F, Briskman J, Frith U. Exploring the cognitive phenotype of autism: weak "central coherence" in parents and siblings of children with autism: I. Experimental tests. Journal of child psychology and psychiatry, and allied disciplines. 2001;42(3):299–307. doi:10.1111/1469-7610.00723. PMID 11321199.
- ↑ Abramson RK, Ravan SA, Wright HH, et al. The relationship between restrictive and repetitive behaviors in individuals with autism and obsessive compulsive symptoms in parents. Child psychiatry and human development. 2005;36(2):155–65. doi:10.1007/s10578-005-2973-7. PMID 16228144.
- ↑ Constantino JN, Todd RD. Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry. 2005;57(6):655–60. doi:10.1016/j.biopsych.2004.12.014. PMID 15780853.
- ↑ Ghaziuddin M. A family history study of Asperger syndrome. Journal of autism and developmental disorders. 2005;35(2):177–82. doi:10.1007/s10803-004-1996-4. PMID 15909404.
- ↑ Greenberg DA, Hodge SE, Sowinski J, Nicoll D. Excess of twins among affected sibling pairs with autism: implications for the etiology of autism. Am J Hum Genet. 2001;69(5):1062–7. doi:10.1086/324191. PMID 11590546.
- ↑ Hallmayer J, Glasson EJ, Bower C, et al. On the twin risk in autism. Am J Hum Genet. 2002;71(4):941–6. doi:10.1086/342990. PMID 12297988.
- ↑ Iossifov, Ivan; O’Roak, Brian J.; Sanders, Stephan J.; Ronemus, Michael; Krumm, Niklas; Levy, Dan; Stessman, Holly A.; Witherspoon, Kali T.; Vives, Laura; Patterson, Karynne E.; Smith, Joshua D.; Paeper, Bryan; Nickerson, Deborah A.; Dea, Jeanselle; Dong, Shan; Gonzalez, Luis E.; Mandell, Jeffrey D.; Mane, Shrikant M.; Murtha, Michael T.; Sullivan, Catherine A.; Walker, Michael F.; Waqar, Zainulabedin; Wei, Liping; Willsey, A. Jeremy; Yamrom, Boris; Lee, Yoon-ha; Grabowska, Ewa; Dalkic, Ertugrul; Wang, Zihua; Marks, Steven; Andrews, Peter; Leotta, Anthony; Kendall, Jude; Hakker, Inessa; Rosenbaum, Julie; Ma, Beicong; Rodgers, Linda; Troge, Jennifer; Narzisi, Giuseppe; Yoon, Seungtai; Schatz, Michael C.; Ye, Kenny; McCombie, W. Richard; Shendure, Jay; Eichler, Evan E.; State, Matthew W.; Wigler, Michael (29 October 2014). "The contribution of de novo coding mutations to autism spectrum disorder". Nature. 515 (7526): 216–221. doi:10.1038/nature13908. PMID 25363768.
- ↑ Gringras, Paul; Chen, Wai (September 2001). "Mechanisms for differences in monozygous twins". Early Human Development. 64 (2): 105–117. doi:10.1016/S0378-3782(01)00171-2. PMID 11440823.
- 1 2 3 Brandler, William M.; Sebat, Jonathan (14 January 2015). "From De Novo Mutations to Personalized Therapeutic Interventions in Autism". Annual Review of Medicine. 66 (1): 487–507. doi:10.1146/annurev-med-091113-024550.
- ↑ Ronemus, Michael; Iossifov, Ivan; Levy, Dan; Wigler, Michael (16 January 2014). "The role of de novo mutations in the genetics of autism spectrum disorders". Nature Reviews Genetics. 15 (2): 133–141. doi:10.1038/nrg3585.
- ↑ "SFARI CNV". https://gene.sfari.org/autdb/CNVHome.do. External link in
|website=(help); - ↑ "SFARI gene scoring module". https://gene.sfari.org/autdb/GS_Home.do. External link in
|website=(help); - 1 2 Levitt P, Campbell DB. The genetic and neurobiologic compass points toward common signaling dysfunctions in autism spectrum disorders. J Clin Invest. 2009;119(4):747–54. doi:10.1172/JCI37934. PMID 19339766.
- 1 2 Shinawi, M.; Liu, P.; Kang, S. H. L.; Shen, J.; Belmont, J. W.; Scott, D. A.; Probst, F. J.; Craigen, W. J.; Graham, B. H.; Pursley, A.; Clark, G.; Lee, J.; Proud, M.; Stocco, A.; Rodriguez, D. L.; Kozel, B. A.; Sparagana, S.; Roeder, E. R.; McGrew, S. G.; Kurczynski, T. W.; Allison, L. J.; Amato, S.; Savage, S.; Patel, A.; Stankiewicz, P.; Beaudet, A. L.; Cheung, S. W.; Lupski, J. R. (12 November 2009). "Recurrent reciprocal 16p11.2 rearrangements associated with global developmental delay, behavioural problems, dysmorphism, epilepsy, and abnormal head size". Journal of Medical Genetics. 47 (5): 332–341. doi:10.1136/jmg.2009.073015.
- 1 2 Moreno-De-Luca, Andres; Myers, Scott M; Challman, Thomas D; Moreno-De-Luca, Daniel; Evans, David W; Ledbetter, David H (April 2013). "Developmental brain dysfunction: revival and expansion of old concepts based on new genetic evidence". The Lancet Neurology. 12 (4): 406–414. doi:10.1016/S1474-4422(13)70011-5.
- ↑ McCarthy, Shane E; Makarov, Vladimir; Kirov, George; Addington, Anjene M; McClellan, Jon; Yoon, Seungtai; Perkins, Diana O; Dickel, Diane E; Kusenda, Mary; Krastoshevsky, Olga; Krause, Verena; Kumar, Ravinesh A; Grozeva, Detelina; Malhotra, Dheeraj; Walsh, Tom; Zackai, Elaine H; Kaplan, Paige; Ganesh, Jaya; Krantz, Ian D; Spinner, Nancy B; Roccanova, Patricia; Bhandari, Abhishek; Pavon, Kevin; Lakshmi, B; Leotta, Anthony; Kendall, Jude; Lee, Yoon-ha; Vacic, Vladimir; Gary, Sydney; Iakoucheva, Lilia M; Crow, Timothy J; Christian, Susan L; Lieberman, Jeffrey A; Stroup, T Scott; Lehtimäki, Terho; Puura, Kaija; Haldeman-Englert, Chad; Pearl, Justin; Goodell, Meredith; Willour, Virginia L; DeRosse, Pamela; Steele, Jo; Kassem, Layla; Wolff, Jessica; Chitkara, Nisha; McMahon, Francis J; Malhotra, Anil K; Potash, James B; Schulze, Thomas G; Nöthen, Markus M; Cichon, Sven; Rietschel, Marcella; Leibenluft, Ellen; Kustanovich, Vlad; Lajonchere, Clara M; Sutcliffe, James S; Skuse, David; Gill, Michael; Gallagher, Louise; Mendell, Nancy R; Craddock, Nick; Owen, Michael J; O'Donovan, Michael C; Shaikh, Tamim H; Susser, Ezra; DeLisi, Lynn E; Sullivan, Patrick F; Deutsch, Curtis K; Rapoport, Judith; Levy, Deborah L; King, Mary-Claire; Sebat, Jonathan (25 October 2009). "Microduplications of 16p11.2 are associated with schizophrenia". Nature Genetics. 41 (11): 1223–1227. doi:10.1038/ng.474. PMC 2951180
 . PMID 19855392.
. PMID 19855392. - ↑ The Simons VIP Consortium (March 2012). "Simons Variation in Individuals Project (Simons VIP): A Genetics-First Approach to Studying Autism Spectrum and Related Neurodevelopmental Disorders". Neuron. 73 (6): 1063–1067. doi:10.1016/j.neuron.2012.02.014.
- ↑ Stefansson, Hreinn; Meyer-Lindenberg, Andreas; Steinberg, Stacy; Magnusdottir, Brynja; Morgen, Katrin; Arnarsdottir, Sunna; Bjornsdottir, Gyda; Walters, G. Bragi; Jonsdottir, Gudrun A.; Doyle, Orla M.; Tost, Heike; Grimm, Oliver; Kristjansdottir, Solveig; Snorrason, Heimir; Davidsdottir, Solveig R.; Gudmundsson, Larus J.; Jonsson, Gudbjorn F.; Stefansdottir, Berglind; Helgadottir, Isafold; Haraldsson, Magnus; Jonsdottir, Birna; Thygesen, Johan H.; Schwarz, Adam J.; Didriksen, Michael; Stensbøl, Tine B.; Brammer, Michael; Kapur, Shitij; Halldorsson, Jonas G.; Hreidarsson, Stefan; Saemundsen, Evald; Sigurdsson, Engilbert; Stefansson, Kari (18 December 2013). "CNVs conferring risk of autism or schizophrenia affect cognition in controls". Nature. 505 (7483): 361–366. doi:10.1038/Nature12818.
- 1 2 Wittkowski KM, Sonakya V, Bigio B, Tonn MK, Shic F, Ascano M, Nasca C, Gold-Von Simson G (January 2014). "A novel computational biostatistics approach implies impaired dephosphorylation of growth factor receptors as associated with severity of autism". Transl Psychiatry. 4: e354. doi:10.1038/tp.2013.124. PMC 3905234
 . PMID 24473445.
. PMID 24473445. - ↑ Zhao, X.; Leotta, A.; Kustanovich, V.; Lajonchere, C.; Geschwind, D. H.; Law, K.; Law, P.; Qiu, S.; Lord, C.; Sebat, J.; Ye, K.; Wigler, M. (25 July 2007). "A unified genetic theory for sporadic and inherited autism". Proceedings of the National Academy of Sciences. 104 (31): 12831–12836. doi:10.1073/pnas.0705803104. PMC 1933261
 . PMID 17652511.
. PMID 17652511. - ↑ Jiang YH, Sahoo T, Michaelis RC, et al. A mixed epigenetic/genetic model for oligogenic inheritance of autism with a limited role for UBE3A. Am J Med Genet A. 2004;131(1):1–10. doi:10.1002/ajmg.a.30297. PMID 15389703.
- ↑ Schanen NC. Epigenetics of autism spectrum disorders. Hum Mol Genet. 2006;15(Review 2):R138–50. doi:10.1093/hmg/ddl213. PMID 16987877.
- ↑ Skuse DH. Imprinting, the X-chromosome, and the male brain: explaining sex differences in the liability to autism. Pediatr Res. 2000;47(1):9–16. doi:10.1203/00006450-200001000-00006. PMID 10625077.
- ↑ Crespi B, Badcock C. Psychosis and autism as diametrical disorders of the social brain. Behav Brain Sci. 2008;31(3):241–61. doi:10.1017/S0140525X08004214. PMID 18578904.
- ↑ Trottier G, Srivastava L, Walker CD. Etiology of infantile autism: a review of recent advances in genetic and neurobiological research. J Psychiatry Neurosci. 1999;24(2):103–115. PMID 10212552.
- ↑ Arndt TL, Stodgell CJ, Rodier PM. The teratology of autism. Int J Dev Neurosci. 2005;23(2–3):189–99. doi:10.1016/j.ijdevneu.2004.11.001. PMID 15749245.
- ↑ Rutter M. Incidence of autism spectrum disorders: changes over time and their meaning. Acta Paediatr. 2005;94(1):2–15. doi:10.1111/j.1651-2227.2005.tb01779.x. PMID 15858952.
- ↑ Szpir M. Tracing the origins of autism: a spectrum of new studies. Environ Health Perspect. 2006;114(7):A412–8. doi:10.1289/ehp.114-a412. PMID 16835042. PMC 1513312.
- ↑ Benvenuto A, Manzi B, Alessandrelli R, Galasso C, Curatolo P. Recent advances in the pathogenesis of syndromic autisms. Int J Pediatr. 2009;2009:198736. doi:10.1155/2009/198736. PMID 19946417. PMC 2778501.
- ↑ Wall DP, Esteban FJ, Deluca TF et al. Comparative analysis of neurological disorders focuses genome-wide search for autism genes. Genomics. 2008;93(2):120–9. doi:10.1016/j.ygeno.2008.09.015. PMID 18950700.
- ↑ Wang K, Zhang H, Ma D et al. Common genetic variants on 5p14.1 associate with autism spectrum disorders [PDF]. Nature. 2009;459(7246):528–33. doi:10.1038/nature07999. PMID 19404256. PMC 2943511. Lay summary: BBC News, 2009-04-28.
- ↑ Pagnamenta, A. T.; Khan, H.; Walker, S.; Gerrelli, D.; Wing, K.; Bonaglia, M. C.; Giorda, R.; Berney, T.; Mani, E.; Molteni, M.; Pinto, D.; Le Couteur, A.; Hallmayer, J.; Sutcliffe, J. S.; Szatmari, P.; Paterson, A. D.; Scherer, S. W.; Vieland, V. J.; Monaco, A. P. (23 October 2010). "Rare familial 16q21 microdeletions under a linkage peak implicate cadherin 8 (CDH8) in susceptibility to autism and learning disability". Journal of Medical Genetics. 48 (1): 48–54. doi:10.1136/jmg.2010.079426.
- 1 2 Brandler, William M.; Antaki, Danny; Gujral, Madhusudan; Noor, Amina; Rosanio, Gabriel; Chapman, Timothy R.; Barrera, Daniel J.; Lin, Guan Ning; Malhotra, Dheeraj; Watts, Amanda C.; Wong, Lawrence C.; Estabillo, Jasper A.; Gadomski, Therese E.; Hong, Oanh; Fajardo, Karin V. Fuentes; Bhandari, Abhishek; Owen, Renius; Baughn, Michael; Yuan, Jeffrey; Solomon, Terry; Moyzis, Alexandra G.; Maile, Michelle S.; Sanders, Stephan J.; Reiner, Gail E.; Vaux, Keith K.; Strom, Charles M.; Zhang, Kang; Muotri, Alysson R.; Akshoomoff, Natacha; Leal, Suzanne M.; Pierce, Karen; Courchesne, Eric; Iakoucheva, Lilia M.; Corsello, Christina; Sebat, Jonathan (March 2016). "Frequency and Complexity of De Novo Structural Mutation in Autism". The American Journal of Human Genetics. 98 (4): 1–13. doi:10.1016/j.ajhg.2016.02.018.
- ↑ Weiss LA, Shen Y, Korn JM et al. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358(7):667–75. doi:10.1056/NEJMoa075974. PMID 18184952. Lay summary: Boston Globe, 2008-01-09.
- ↑ Marshall CR, Noor A, Vincent JB et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82(2):477–88. doi:10.1016/j.ajhg.2007.12.009. PMID 18252227. PMC 2426913. Lay summary: MedPage Today, 2008-01-17.
- ↑ Samuels IS, Saitta SC, Landreth GE. MAP'ing CNS development and cognition: an ERKsome process. Neuron. 2009;61(2):160–7. doi:10.1016/j.neuron.2009.01.001. PMID 19186160. Lay summary: ScienceDaily, 2009-02-12.
- ↑ Surtees PG, Wainwright NW, Willis-Owen SA, Luben R, Day NE, Flint J. Social adversity, the serotonin transporter (5-HTTLPR) polymorphism and major depressive disorder. Biol Psychiatry. 2006;59(3):224–9. doi:10.1016/j.biopsych.2005.07.014. PMID 16154545.
- ↑ Sutcliffe JS, Delahanty RJ, Prasad HC, et al. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am J Hum Genet. 2005;77(2):265–79. doi:10.1086/432648. PMID 15995945.
- ↑ Devlin B, Cook EH, Coon H, et al. Autism and the serotonin transporter: the long and short of it. Mol Psychiatry. 2005;10(12):1110–6. doi:10.1038/sj.mp.4001724. PMID 16103890.
- ↑ Coutinho AM, Oliveira G, Morgadinho T, et al. Variants of the serotonin transporter gene (SLC6A4) significantly contribute to hyperserotonemia in autism. Mol Psychiatry. 2004;9(3):264–71. doi:10.1038/sj.mp.4001409. PMID 15094787.
- ↑ Huang CH, Santangelo SL. Autism and serotonin transporter gene polymorphisms: a systematic review and meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(6):903–13. doi:10.1002/ajmg.b.30720. PMID 18286633.
- ↑ Strom SP, Stone JL, ten Bosch JR et al. High-density SNP association study of the 17q21 chromosomal region linked to autism identifies CACNA1G as a novel candidate gene. Mol Psychiatry. 2009;15(10):996–1005. doi:10.1038/mp.2009.41. PMID 19455149. Lay summary: Press release, 2009-05-19.
- ↑ Ma DQ, Whitehead PL, Menold MM, et al. Identification of significant association and gene-gene interaction of GABA receptor subunit genes in autism. Am J Hum Genet. 2005;77(3):377–88. doi:10.1086/433195. PMID 16080114.
- ↑ Nurmi EL, Dowd M, Tadevosyan-Leyfer O, Haines JL, Folstein SE, Sutcliffe JS. Exploratory subsetting of autism families based on savant skills improves evidence of genetic linkage to 15q11-q13. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42(7):856–63. doi:10.1097/01.CHI.0000046868.56865.0F. PMID 12819446.
- ↑ Delorey TM, Sahbaie P, Hashemi E, Homanics GE, Clark JD. Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non-selective attention and hypoplasia of cerebellar vermal lobules: A potential model of autism spectrum disorder. Behav Brain Res. 2007;187(2):207–20. doi:10.1016/j.bbr.2007.09.009. PMID 17983671.
- ↑ Benayed R, Gharani N, Rossman I, et al. Support for the homeobox transcription factor gene ENGRAILED 2 as an autism spectrum disorder susceptibility locus. Am J Hum Genet. 2005;77(5):851–68. doi:10.1086/497705. PMID 16252243.
- ↑ Zhong H, Serajee FJ, Nabi R, Huq AH. No association between the EN2 gene and autistic disorder. J Med Genet. 2003;40(1):e4. doi:10.1136/jmg.40.1.e4. PMID 12525552.
- ↑ Auranen M, Varilo T, Alen R, et al. Evidence for allelic association on chromosome 3q25-27 in families with autism spectrum disorders originating from a subisolate of Finland. Mol Psychiatry. 2003;8(10):879–84. doi:10.1038/sj.mp.4001299. PMID 14515138.
- ↑ Ylisaukko-oja T, Nieminen-von Wendt T, Kempas E, et al. Genome-wide scan for loci of Asperger syndrome. Mol Psychiatry. 2004;9(2):161–8. doi:10.1038/sj.mp.4001385. PMID 14966474.
- ↑ Auranen M, Vanhala R, Varilo T, et al. A genomewide screen for autism-spectrum disorders: evidence for a major susceptibility locus on chromosome 3q25-27. Am J Hum Genet. 2002;71(4):777–90. doi:10.1086/342720. PMID 12192642.
- ↑ Serajee FJ, Zhong H, Mahbubul Huq AH. Association of Reelin gene polymorphisms with autism. Genomics. 2006;87(1):75–83. doi:10.1016/j.ygeno.2005.09.008. PMID 16311013.
- ↑ Skaar DA, Shao Y, Haines JL, et al. Analysis of the RELN gene as a genetic risk factor for autism. Mol Psychiatry. 2005;10(6):563–71. doi:10.1038/sj.mp.4001614. PMID 15558079.
- ↑ Li J, Nguyen L, Gleason C, et al. Lack of evidence for an association between WNT2 and RELN polymorphisms and autism. Am J Med Genet B Neuropsychiatr Genet. 2004;126(1):51–7. doi:10.1002/ajmg.b.20122. PMID 15048648.
- ↑ Segurado R, Conroy J, Meally E, Fitzgerald M, Gill M, Gallagher L. Confirmation of association between autism and the mitochondrial aspartate/glutamate carrier SLC25A12 gene on chromosome 2q31. The American Journal of Psychiatry. 2005;162(11):2182–4. doi:10.1176/appi.ajp.162.11.2182. PMID 16263864.
- ↑ Ramoz N, Reichert JG, Smith CJ, et al. Linkage and association of the mitochondrial aspartate/glutamate carrier SLC25A12 gene with autism. The American Journal of Psychiatry. 2004;161(4):662–9. doi:10.1176/appi.ajp.161.4.662. PMID 15056512.
- ↑ Lepagnol-Bestel AM, Maussion G, Boda B et al. SLC25A12 expression is associated with neurite outgrowth and is upregulated in the prefrontal cortex of autistic subjects. Mol Psychiatry. 2008;13(4):385–97. doi:10.1038/sj.mp.4002120. PMID 18180767.
- ↑ Blasi F, Bacchelli E, Carone S, et al. SLC25A12 and CMYA3 gene variants are not associated with autism in the IMGSAC multiplex family sample. Eur J Hum Genet. 2006;14(1):123–6. doi:10.1038/sj.ejhg.5201444. PMID 16205742.
- ↑ Kent L, Gallagher L, Elliot HR, Mowbray C, Chinnery PF. An investigation of mitochondrial haplogroups in autism. Am J Med Genet B Neuropsychiatr Genet. 2007;147B(6):987–9. doi:10.1002/ajmg.b.30687. PMID 18161860.
- ↑ Rodier PM. The early origins of autism. Sci Am. 2000;282(2):56–63. doi:10.1038/scientificamerican0200-56. PMID 10710787.
- ↑ Conciatori M, Stodgell CJ, Hyman SL, et al. Association between the HOXA1 A218G polymorphism and increased head circumference in patients with autism. Biol Psychiatry. 2004;55(4):413–9. doi:10.1016/j.biopsych.2003.10.005. PMID 14960295.
- ↑ Gallagher L, Hawi Z, Kearney G, Fitzgerald M, Gill M. No association between allelic variants of HOXA1/HOXB1 and autism. Am J Med Genet B Neuropsychiatr Genet. 2004;124(1):64–7. doi:10.1002/ajmg.b.20094. PMID 14681917.
- ↑ Collins JS, Schroer RJ, Bird J, Michaelis RC. The HOXA1 A218G polymorphism and autism: lack of association in white and black patients from the South Carolina Autism Project. Journal of autism and developmental disorders. 2003;33(3):343–8. doi:10.1023/A:1024414803151. PMID 12908836.
- ↑ Talebizadeh Z, Bittel DC, Miles JH, et al. No association between HOXA1 and HOXB1 genes and autism spectrum disorders (ASD). J Med Genet. 2002;39(11):e70. doi:10.1136/jmg.39.11.e70. PMID 12414832.
- ↑ Tischfield MA, Bosley TM, Salih MA, et al. Homozygous HOXA1 mutations disrupt human brainstem, inner ear, cardiovascular and cognitive development. Nat Genet. 2005;37(10):1035–7. doi:10.1038/ng1636. PMID 16155570.
- ↑ Ingram JL, Stodgell CJ, Hyman SL, Figlewicz DA, Weitkamp LR, Rodier PM. Discovery of allelic variants of HOXA1 and HOXB1: genetic susceptibility to autism spectrum disorders. Teratology. 2000;62(6):393–405. doi:10.1002/1096-9926(200012)62:6<393::AID-TERA6>3.0.CO;2-V. PMID 11091361.
- ↑ Rossel M, Capecchi MR. Mice mutant for both Hoxa1 and Hoxb1 show extensive remodeling of the hindbrain and defects in craniofacial development. Development. 1999;126(22):5027–40. PMID 10529420.
- ↑ In utero exposure to valproic acid and autism--a current review of clinical and animal studies. Neurotoxicology and teratology. 2013;36:47–56. doi:10.1016/j.ntt.2013.01.004. PMID 23395807.
- ↑ Philippi A, Roschmann E, Tores F, et al. Haplotypes in the gene encoding protein kinase c-beta (PRKCB1) on chromosome 16 are associated with autism. Mol Psychiatry. 2005;10(10):950–60. doi:10.1038/sj.mp.4001704. PMID 16027742.
- ↑ Medina JJ. Fishing for genetic links in autism. Psychiatr Times. 2009;26(3).
- 1 2 Hogart A, Wu D, Lasalle JM, Schanen NC. The comorbidity of autism with the genomic disorders of chromosome 15q11.2-q13. Neurobiol Dis. 2008;38(2):181–91. doi:10.1016/j.nbd.2008.08.011. PMID 18840528.
- ↑ Schuetz G, Rosário M, Grimm J, Boeckers TM, Gundelfinger ED, Birchmeier W. The neuronal scaffold protein Shank3 mediates signaling and biological function of the receptor tyrosine kinase Ret in epithelial cells. J Cell Biol. 2004;167(5):945–52. doi:10.1083/jcb.200404108. PMID 15569713. PMC 2172453.
- 1 2 3 Deletion 22q13 Syndrome M.C Phelan (2003) Orphanet.com
- 1 2 Durand CM, Betancur C, Boeckers TM, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39(1):25–7. doi:10.1038/ng1933. PMID 17173049. Lay summary: Autism Speaks.
- ↑ Neuroligins Kristen Harris (2001) Cell adhesion at synapses Synapse Web, Laboratory of Synapse Structure and Function. Human Brain Project. National Institute of Mental Health and the National Institute of Drug Abuse
- ↑ Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119(7):1013–26. doi:10.1016/j.cell.2004.11.035. PMID 15620359.
- ↑ Tabuchi K, Blundell J, Etherton MR et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318(5847):71–6. doi:10.1126/science.1146221. PMID 17823315. Lay summary: Science Daily, 2007-09-08.
- ↑ Wermter AK, Kamp-Becker I, Strauch K, Schulte-Körne G, Remschmidt H. No evidence for involvement of genetic variants in the X-linked neuroligin genes NLGN3 and NLGN4X in probands with autism spectrum disorder on high functioning level. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(4):535–7. doi:10.1002/ajmg.b.30618. PMID 18189281.
- 1 2 Gene Linked to Autism in Families with More Than One Affected Child National Institutes of Health News (2006) Retrieved March 3, 2007
- ↑ Campbell DB, Sutcliffe JS, Ebert PJ, et al. A genetic variant that disrupts MET transcription is associated with autism. Proc Natl Acad Sci USA. 2006;103(45):16834–9. doi:10.1073/pnas.0605296103. PMID 17053076. Lay summary: BBC News, 2006-10-28.
- ↑ Kim HG, Kishikawa S, Higgins AW et al. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008;82(1):199–207. doi:10.1016/j.ajhg.2007.09.011. PMID 18179900. PMC 2253961.
- ↑ Alarcón M, Abrahams BS, Stone JL et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82(1):150–9. doi:10.1016/j.ajhg.2007.09.005. PMID 18179893. PMC 2253955. Lay summary: UCLA Newsroom, 2008-01-10.
- ↑ Arking DE, Cutler DJ, Brune CW et al. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet. 2008;82(1):160–4. doi:10.1016/j.ajhg.2007.09.015. PMID 18179894. PMC 2253968. Lay summary: Johns Hopkins Medicine, 2008-01-22.
- ↑ Bakkaloglu B, O'Roak BJ, Louvi A et al. Molecular cytogenetic analysis and resequencing of Contactin Associated Protein-Like 2 in autism spectrum disorders. Am J Hum Genet. 2008;82(1):165–73. doi:10.1016/j.ajhg.2007.09.017. PMID 18179895. PMC 2253974.
- ↑ Marui T, Koishi S, Funatogawa I, et al. No association of FOXP2 and PTPRZ1 on 7q31 with autism from the Japanese population. Neurosci Res. 2005;53(1):91–4. doi:10.1016/j.neures.2005.05.003. PMID 15998549.
- ↑ Gauthier J, Joober R, Mottron L, et al. Mutation screening of FOXP2 in individuals diagnosed with autistic disorder. Am J Med Genet A. 2003;118(2):172–5. doi:10.1002/ajmg.a.10105. PMID 12655497.
- ↑ Vernes SC, Newbury DF, Abrahams BS et al. A functional genetic link between distinct developmental language disorders. N Engl J Med. 2008;359(22):2337–45. doi:10.1056/NEJMoa0802828. PMID 18987363. Lay summary: Science News, 2008-11-05.
- ↑ Williams TA, Mars AE, Buyske SG, et al. Risk of autistic disorder in affected offspring of mothers with a glutathione S-transferase P1 haplotype. Archives of pediatrics & adolescent medicine. 2007;161(4):356–61. doi:10.1001/archpedi.161.4.356. PMID 17404132.
- ↑ LoParo, D; Waldman, I D (5 August 2014). "The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: a meta-analysis". Molecular Psychiatry. doi:10.1038/mp.2014.77.
- 1 2 Ylisaukko-oja T, Alarcón M, Cantor RM, et al. Search for autism loci by combined analysis of Autism Genetic Resource Exchange and Finnish families. Ann Neurol. 2006;59(1):145–55. doi:10.1002/ana.20722. PMID 16288458.
- ↑ Lauritsen MB, Als TD, Dahl HA, et al. A genome-wide search for alleles and haplotypes associated with autism and related pervasive developmental disorders on the Faroe Islands. Mol Psychiatry. 2006;11(1):37–46. doi:10.1038/sj.mp.4001754. PMID 16205737.
- ↑ Trikalinos TA, Karvouni A, Zintzaras E, et al. A heterogeneity-based genome search meta-analysis for autism-spectrum disorders. Mol Psychiatry. 2006;11(1):29–36. doi:10.1038/sj.mp.4001750. PMID 16189507.
- ↑ Yonan AL, Alarcón M, Cheng R, et al. A genomewide screen of 345 families for autism-susceptibility loci. Am J Hum Genet. 2003;73(4):886–97. doi:10.1086/378778. PMID 13680528.
- ↑ Cantor RM, Kono N, Duvall JA, et al. Replication of autism linkage: fine-mapping peak at 17q21. Am J Hum Genet. 2005;76(6):1050–6. doi:10.1086/430278. PMID 15877280.
- ↑ Butler MG, Dasouki MJ, Zhou XP, et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet. 2005;42(4):318–21. doi:10.1136/jmg.2004.024646. PMID 15805158.
- ↑ Bearden, Carrie (2016-10-18). "Same DNA deletion paves paths to autism, schizophrenia". Spectrum. Retrieved 2016-10-18.
- ↑ Morrow EM, Yoo S, Flavell SW et al. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321(5886):218–23. doi:10.1126/science.1157657. PMID 18621663. Lay summary: The Times, 2008-07-11.
- ↑ Geschwind DH. Autism: Family connections. Nature. 2008;454(7206):838–9. doi:10.1038/454838a. PMID 18704077.
- ↑ Flavell SW, Cowan CW, Kim T, et al. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311(5763):1008–12. doi:10.1126/science.1122511. PMID 16484497.
- ↑ Li H, Radford JC, Ragusa MJ, et al. Transcription factor MEF2C influences neural stem/progenitor cell differentiation and maturation in vivo. Proc Natl Acad Sci USA. 2008;105(27):9397–402. doi:10.1073/pnas.0802876105. PMID 18599437.
Further reading
- O'Roak BJ, State MW. Autism genetics: strategies, challenges, and opportunities. Autism Res. 2008;1(1):4–17. doi:10.1002/aur.3. PMID 19360646. An excellent summary of the state and possible future directions of autism genetics research as of late 2007.
- Fisch GS. Syndromes and epistemology II: is autism a polygenic disorder? |title.=. Am J Med Genet A. 2008;146A(17):2203–12. doi:10.1002/ajmg.a.32438. PMID 18666231. Advocates common disease rare allele (CDRA) over polygenic approaches.
- Losh M, Sullivan PF, Trembath D, Piven J. Current developments in the genetics of autism: from phenome to genome. J Neuropathol Exp Neurol. 2008;67(9):829–37. doi:10.1097/NEN.0b013e318184482d. PMID 18716561. Focuses on attempts to match genes to behavior.
External links
- Autism Genetic Resource Exchange (AGRE) - 'the world's first collaborative gene bank for autism'