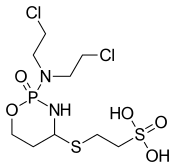
Mafosfamide
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-{(2-[bis(2-chloroethyl)amino]-2-oxido-1,3,2-oxazaphosphinan-4-yl}thio)ethanesulfonic acid | |
| Other names
2-{[2-[bis(2-chloroethyl)amino]-2-oxo-1-oxa-3-aza-2λ5-phosphacyclohex-4-yl}sulfanyl]ethanesulfonic acid | |
| Identifiers | |
| 88859-04-5 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 16736958 |
| MeSH | Mafosfamide |
| PubChem | 104746 |
| UNII | 5970HH9923 |
| |
| |
| Properties | |
| C9H19Cl2N2O5PS2 | |
| Molar mass | 401.269 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Mafosfamide (INN) is an oxazaphosphorine (cyclophosphamide-like) alkylating agent under investigation as a chemotherapeutic. It is metabolized by cytochrome P450 into 4-hydroxycyclophosphamide, which is then converted into aldophosphamide, which, in turn yields the cytotoxic metabolites phosphoramide mustard and acrolein.[1]
Several Phase I trials have been completed.[2][3]
References
- ↑ Ludeman SM. The chemistry of the metabolites of cyclophosphamide. Curr Pharm Des. 1999 Aug;5(8):627-43. PMID 10469895
- ↑ "Intrathecal Mafosfamide". ClinicalTrials.gov. U.S. National Institutes of Health. August 21, 2006. Retrieved 2007-07-13. ClinicalTrials.gov Identifier NCT00062881.
- ↑ "Mafosfamide in Treating Patients With Progressive or Refractory Meningeal Tumors". ClinicalTrials.gov. U.S. National Institutes of Health. February 20, 2007. Retrieved 2007-07-13. ClinicalTrials.gov Identifier NCT00031928.
This article is issued from Wikipedia - version of the 8/30/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.