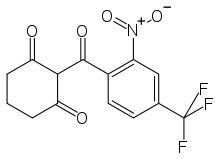
Nitisinone
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| License data |
|
| Routes of administration | Oral |
| ATC code | A16AX04 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Biological half-life | Approximately 54 h |
| Identifiers | |
| |
| CAS Number |
104206-65-7 |
| PubChem (CID) | 115355 |
| IUPHAR/BPS | 6834 |
| DrugBank |
DB00348 |
| ChemSpider |
103195 |
| UNII |
K5BN214699 |
| KEGG |
D05177 |
| ChEBI |
CHEBI:50378 |
| ChEMBL |
CHEMBL1337 |
| ECHA InfoCard | 100.218.521 |
| Chemical and physical data | |
| Formula | C14H10F3NO5 |
| Molar mass | 329.228 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Nitisinone (INN), also known as NTBC (an abbreviation of its full chemical name) is a medication used to slow the effects of hereditary tyrosinemia type 1. Since its first use for this indication in 1991, it has replaced liver transplantation as the first-line treatment for this rare condition. It is also being studied in the related condition alkaptonuria. It is marketed under the brand name Orfadin by the company Swedish Orphan Biovitrum (Sobi); it was first brought to market by Swedish Orphan International. It was originally developed as a candidate herbicide.
Uses
Nitisinone is used to treat hereditary tyrosinemia type 1, in combination with restriction of tyrosine in the diet.[1][2][3]
Since its first use for this indication in 1991, it has replaced liver transplantation as the first-line treatment for this rare condition.[4] I It is marketed under the brand name Orfadin.
It has been demonstrated that treatment with nitisinone can reduce urinary levels of homogentisic acid in alkaptonuria patients by 95%.[5] A series of clinical trials run by DevelopAKUre to determine whether nitisinone is effective at treating the ochronosis suffered by patients with alkaptonuria are ongoing.[6] If the trials are successful, DevelopAKUre will try to get nitisinone licensed for use by alkaptonuria patients.[7]
Mechanism of action
The mechanism of action of nitisinone involves reversibile inhibition of 4-Hydroxyphenylpyruvate dioxygenase (HPPD),.[8][9] This is a treatment for patients with Tyrosinemia type 1 as it prevents the formation of maleylacetoacetic acid and fumarylacetoacetic acid, which have the potential to be converted to succinyl acetone, a toxin that damages the liver and kidneys.[4] This causes the symptoms of Tyrosinemia type 1 experienced by untreated patients.[10]
Alkaptonuria is caused when an enzyme called homogentisic dioxygenase (HGD) is faulty so can't break down homogentisic acid (HGA).[11] Alkaptonuria patients treated with nitisinone produce far less HGA than those not treated (95% less in the urine),[5] because nitisinone inhibits HPPD meaning HGA doesn't form in the first place. Clinical trials are ongoing to test whether nitisinone could prevent the ochronosis experienced by older alkaptonuria patients.[6]
Adverse effects
Nitisinone has several negative side effects; these include but are not limited to: bloated abdomen, dark urine, abdominal pain, feeling of tiredness or weakness, headache, light-colored stools, loss of appetite, weight loss, vomiting, and yellow-colored eyes or skin.[12]
Research
Nitisinone is being studied as a treatment for alkaptonuria.[13]
Research at the National Institutes of Health (NIH) has demonstrated that nitisinone can reduce urinary levels of HGA by up to 95% in patients with alkaptonuria. The primary parameter of the NIH trial was range of hip motion, for which the results were inconclusive.
Research done using alkaptonuric mice has shown that mice treated with nitisinone experience no ochronosis in knee joint cartilage. In contrast, all of the mice in the untreated control group developed ochronotic knee joints.[14]
The efficacy of Nitisinone is now being studied in a series international clinical trials called DevelopAKUre.[15] The studies will recruit alkaptonuria patients in Europe.[16] A larger number of patients will be recruited in these trials than in the previous NIH trial.[17] The trials are funded by the European Commission.[18]
Nitisinone has been shown to increase skin and eye pigmentation in mice, and has been suggested as a possible treatment for oculocutaneous albinism.[19][20]
History
Nitisinone was discovered as part of a program to develop a class of herbicides called HPPD inhibitors. It is a member of the benzoylcyclohexane-1,3-dione family of herbicides, which are chemically derived from a natural phytotoxin obtained from the Australian bottlebrush plant, Callistemon citrinus.[21] HPPD is essential in plants and animals for catabolism, or breaking apart, of tyrosine.[22] In plants, preventing this process leads to destruction of chlorophyll and the death of the plant.[22] In toxicology studies of the herbicide, it was discovered that it had activity against HPPD in rats[23] and humans.[24]
In Type I tyrosinemia, a different enzyme involved in the breakdown of tyrosine, fumarylacetoacetate hydrolase is mutated and doesn't work, leading to very harmful products building up in the body.[1] Fumarylacetoacetate hydrolase acts on tyrosine after HPPD does, so scientists working on making herbicides in the class of HPPD inhibitors hypothesized that inhibiting HPPD and controlling tyrosine in the diet could treat this disease. A series of small clinical trials attempted with one of their compounds, nitisinone, were conducted and were successful, leading to nitisinone being brought to market as an orphan drug Swedish Orphan International,[8] which was later acquired by Swedish Orphan Biovitrum (Sobi).
Sobi is now a part of the DevelopAKUre consortium. They are responsible for drug supply and regulatory support in the ongoing clinical trials that will test the efficiacy of nitisinone as a treatment for alkaptonuria.[25] It is hoped that if the trials are successful, nitisinone could also be licensed for treatment of alkaptonuria.[7]
References
- 1 2 National Organization for Rare Disorders. Physician’s Guide to Tyrosinemia Type 1
- ↑ "Nitisinone (Oral Route) Description and Brand Names". Mayoclinic.com. 2015-04-01. Retrieved 2015-06-04.
- ↑ Sobi Orfadin® (nitisinone)
- 1 2 McKiernan, Patrick J (2006). "Nitisinone in the Treatment of Hereditary Tyrosinaemia Type 1". Drugs. 66 (6): 743–50. doi:10.2165/00003495-200666060-00002. PMID 16706549.
- 1 2 Introne, Wendy J.; Perry, Monique B.; Troendle, James; Tsilou, Ekaterini; Kayser, Michael A.; Suwannarat, Pim; O'Brien, Kevin E.; Bryant, Joy; Sachdev, Vandana; Reynolds, James C.; Moylan, Elizabeth; Bernardini, Isa; Gahl, William A. (2011). "A 3-year randomized therapeutic trial of nitisinone in alkaptonuria". Molecular Genetics and Metabolism. 103 (4): 307–14. doi:10.1016/j.ymgme.2011.04.016. PMID 21620748.
- 1 2 "About DevelopAKUre | DevelopAKUre". Developakure.eu. 2014-06-20. Retrieved 2015-06-04.
- 1 2 "A Potential Drug – Nitisinone". Akusociety.org. Retrieved 2015-06-04.
- 1 2 Lock, E. A.; Ellis, M. K.; Gaskin, P.; Robinson, M.; Auton, T. R.; Provan, W. M.; Smith, L. L.; Prisbylla, M. P.; Mutter, L. C.; Lee, D. L. (1998). "From toxicological problem to therapeutic use: The discovery of the mode of action of 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC), its toxicology and development as a drug". Journal of Inherited Metabolic Disease. 21 (5): 498–506. doi:10.1023/A:1005458703363. PMID 9728330.
- ↑ Kavana, Michael; Moran, Graham R. (2003). "Interaction of (4-Hydroxyphenyl)pyruvate Dioxygenase with the Specific Inhibitor 2-[2-Nitro-4-(trifluoromethyl)benzoyl]-1,3-cyclohexanedione†". Biochemistry. 42 (34): 10238–45. doi:10.1021/bi034658b. PMID 12939152.
- ↑ "Newborn Screening". Newbornscreening.info. 2013-05-14. Retrieved 2015-06-04.
- ↑ "What is Alkaptonuria?". Akusociety.org. Retrieved 2015-06-04.
- ↑ "Nitisinone (Oral Route) Side Effects". Mayoclinic.com. 2015-04-01. Retrieved 2015-06-04.
- ↑ Phornphutkul, Chanika; Introne, Wendy J.; Perry, Monique B.; Bernardini, Isa; Murphey, Mark D.; Fitzpatrick, Diana L.; Anderson, Paul D.; Huizing, Marjan; Anikster, Yair; Gerber, Lynn H.; Gahl, William A. (2002). "Natural History of Alkaptonuria". New England Journal of Medicine. 347 (26): 2111–21. doi:10.1056/NEJMoa021736. PMID 12501223.
- ↑ Preston, A. J.; Keenan, C. M.; Sutherland, H.; Wilson, P. J.; Wlodarski, B.; Taylor, A. M.; Williams, D. P.; Ranganath, L. R.; Gallagher, J. A.; Jarvis, J. C. (2013). "Ochronotic osteoarthropathy in a mouse model of alkaptonuria, and its inhibition by nitisinone". Annals of the Rheumatic Diseases. 73 (1): 284–9. doi:10.1136/annrheumdis-2012-202878. PMID 23511227.
- ↑ "DevelopAKUre". Developakure.eu. 2014-06-20. Retrieved 2015-06-04.
- ↑ "2012-005340-24". Clinicaltrialsregister.eu. Retrieved 2015-06-04.
- ↑ "The Programme | DevelopAKUre". Developakure.eu. 2014-06-20. Retrieved 2015-06-04.
- ↑ "European Commission : CORDIS : Search : Simple". Cordis.europa.eu. 2012-05-30. Retrieved 2015-06-04.
- ↑ Onojafe, Ighovie F.; Adams, David R.; Simeonov, Dimitre R.; Zhang, Jun; Chan, Chi-Chao; Bernardini, Isa M.; Sergeev, Yuri V.; Dolinska, Monika B.; Alur, Ramakrishna P.; Brilliant, Murray H.; Gahl, William A.; Brooks, Brian P. (2011). "Nitisinone improves eye and skin pigmentation defects in a mouse model of oculocutaneous albinism". Journal of Clinical Investigation. 121 (10): 3914–23. doi:10.1172/JCI59372. PMID 21968110. Lay summary – ScienceDaily (September 26, 2011).
- ↑ "Nitisinone for Type 1B Oculocutaneous Albinism - Full Text View". ClinicalTrials.gov. Retrieved 2015-06-04.
- ↑ G. Mitchell, D.W. Bartlett, T.E. Fraser, T.R. Hawkes, D.C. Holt, J.K. Townson, R.A. Wichert Mesotrione: a new selective herbicide for use in maize Pest Management Science, 57 (2) (2001), pp. 120–128
- 1 2 Moran, Graham R. (2005). "4-Hydroxyphenylpyruvate dioxygenase". Archives of Biochemistry and Biophysics. 433 (1): 117–28. doi:10.1016/j.abb.2004.08.015. PMID 15581571.
- ↑ Ellis, M.K.; Whitfield, A.C.; Gowans, L.A.; Auton, T.R.; Provan, W.M.; Lock, E.A.; Smith, L.L. (1995). "Inhibition of 4-Hydroxyphenylpyruvate Dioxygenase by 2-(2-Nitro-4-trifluoromethylbenzoyl)-cyclohexane-1,3-dione and 2-(2-Chloro-4-methanesulfonylbenzoyl)-cyclohexane-1,3-dione". Toxicology and Applied Pharmacology. 133 (1): 12–9. doi:10.1006/taap.1995.1121. PMID 7597701.
- ↑ Lindstedt, Sven; Odelhög, Birgit (1987). "4-Hydroxyphenylpyruvate dioxygenase from human liver". In Kaufman, Seymour. Metabolism of Aromatic Amino Acids and Amines. Methods in Enzymology. 142. pp. 139–42. doi:10.1016/S0076-6879(87)42021-1. ISBN 978-0-12-182042-8. PMID 3037254.
- ↑ "Others | DevelopAKUre". Developakure.eu. 2014-06-20. Retrieved 2015-06-04.