1,4-Benzoquinone
| |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name
Cyclohexa-2,5-diene-1,4-dione | |||
| Other names
p-benzoquinone; p-quinone | |||
| Identifiers | |||
| 106-51-4 | |||
| 3D model (Jmol) | Interactive image Interactive image | ||
| ChEBI | CHEBI:16509 | ||
| ChEMBL | ChEMBL8320 | ||
| ChemSpider | 4489 | ||
| ECHA InfoCard | 100.003.097 | ||
| 6307 | |||
| KEGG | C00472 | ||
| PubChem | 4650 | ||
| RTECS number | DK2625000 | ||
| UNII | 3T006GV98U | ||
| |||
| |||
| Properties | |||
| C6H4O2 | |||
| Molar mass | 108.10 g·mol−1 | ||
| Appearance | Yellow solid | ||
| Odor | Acrid, chlorine-like[1] | ||
| Density | 1.318 g/cm3 at 20 °C | ||
| Melting point | 115 °C (239 °F; 388 K) | ||
| Boiling point | Sublimes | ||
| Slightly soluble | |||
| Solubility | Slightly soluble in petroleum ether; soluble in acetone; very soluble in ethanol, benzene, diethyl ether | ||
| Vapor pressure | 0.1 mmHg (25°C)[1] | ||
| Hazards | |||
| Main hazards | Toxic | ||
| R-phrases | R23/25 R36/37/38 R50 | ||
| S-phrases | S26 S28 S45 S61 | ||
| Flash point | 38 to 93 °C; 100 to 200 °F; 311 to 366 K[1] | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) |
296 mg/kg (mammal, subcutaneous) 93.8 mg/kg (mouse, subcutaneous) 8.5 mg/kg (mouse, IP) 5.6 mg/kg (rat) 130 mg/kg (rat, oral) 25 mg/kg (rat, IV)[2] | ||
| US health exposure limits (NIOSH): | |||
| PEL (Permissible) |
TWA 0.4 mg/m3 (0.1 ppm)[1] | ||
| REL (Recommended) |
TWA 0.4 mg/m3 (0.1 ppm)[1] | ||
| IDLH (Immediate danger) |
100 mg/m3[1] | ||
| Related compounds | |||
| Related compounds |
1,2-Benzoquinone | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone.[3] The molecule is multifunctional: it exhibits properties of a ketone, forming an oxime; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound.
Preparation
Industrial routes
1,4-Benzoquinone is prepared by oxidation of diisopropylbenzene via a reaction related to the Hock rearrangement:
- C6H4(CHMe2)2 + 3 O2 → C6H4O2 + 2 OCMe2 + H2O
The reaction proceeds via the bis(hydroperoxide). Acetone is a coproduct.[4]
Another major process involves the direct hydroxylation of phenol by acidic hydrogen peroxide: C6H5OH + H2O2 → C6H4(OH)2 + H2O Both hydroquinone and catechol are produced. Subsequent oxidation of the hydroquinone gives the quinone.[5]
Quinone was originally prepared industrially by oxidation of aniline, for example by manganese dioxide.[6] This method is mainly practiced in PRC where environmental regulations are more relaxed.
Laboratory routes
The oxidation of hydroquinone is rapid and convenient and therefore desirable for the laboratory. 1,4-Benzoquinone can be prepared from hydroquinone via a number of oxidation methods.[3][7][8] One such method makes use of hydrogen peroxide as the oxidizer and iodine or an iodine salt as a catalyst for the oxidation occurring in a polar solvent; e.g. isopropyl alcohol.[9]
When heated to its melting point, the product sublimates at atmospheric pressure, and, when prepared from hydroquinone, the substrate boils at a significantly higher temperature than 1,4-benzoquinones melting point, allowing for an effective separation of the two. Impure samples are often dark-colored due to the presence of quinhydrone (1:1 complex of quinone with hydroquinone). When prepared by methods involving iodine (an element with a characteristically intense stain), traces of iodine contamination in the product may also darken its appearance. 1,4-Benzoquinone's high vapour pressure at room temperature means that containers should be well sealed to prevent its gradual sublimation into the atmosphere whilst in storage.
Structure and redox
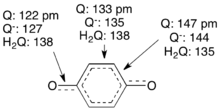
Benzoquinone is a planar molecule with localized, alternating C=C, C=O, and C–C bonds. Reduction gives the semiquinone anion C6H4O2−, which adopts a more delocalized structure. Further reduction coupled to protonation gives the hydroquinone, wherein the C6 ring is fully delocalized.[10]
Applications
Quinone is mainly used as a precursor to hydroquinone, which is used in photography and rubber manufacture as a reducing agent and antioxidant.[5] Benzoquinonium is a Skeletal muscle relaxant, ganglion blocking agent that is made from benzoquinone.[11]
Organic synthesis
It is used as a hydrogen acceptor and oxidant in organic synthesis.[12] 1,4-Benzoquinone serves as a dehydrogenation reagent. It is also used as a dienophile in Diels Alder reactions.[13]
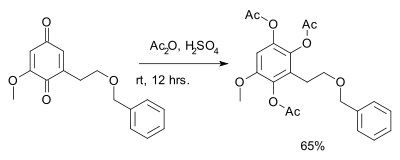
Benzoquinone reacts with acetic anhydride and sulfuric acid to give the triacetate of hydroxyquinol.[14][15] This reaction is called the Thiele reaction or Thiele–Winter reaction[16][17] after Johannes Thiele, who first described it in 1898, and after Ernst Winter, who further described its reaction mechanism in 1900. An application is found in total synthesis:[18]
Benzoquinone is also used to suppress double-bond migration during olefin metathesis reactions.
An acidic potassium iodide solution reduces a solution of benzoquinone to hydroquinone, which can be reoxidized back to the quinone with a solution of silver nitrate.
Due to its ability to function as an oxidizer, 1,4-benzoquinone can be found in methods using the Wacker-Tsuji oxidation, wherein a palladium salt functions to catalytically oxidize an alkene to its corresponding ketone. This reaction is typically carried out using pressurized oxygen as the oxidizer, and can be done instead with methyl nitrite in solution, but benzoquinone can sometimes be preferential, as it does not require the handling of gases, making it easy to measure out by mass.
1,4-Benzoquinone is used in the synthesis of Bromadol and related analogs. It is also used as a reagent in the "Wacker oxidation" reaction. An interesting analog of fentanyl was also made that used the 1,4-Benzoquinone as the starting material. Another analog based on tramadol also appeared in the patent literature, with 4,4-hydroxy(β-phenethyl) substitution pattern. All the drug molecules are made only after the two olefinic double bonds have been reduced.
Metabolism
1,4-Benzoquinone is a toxic metabolite found in human blood and can be used to track exposure to benzene or mixtures containing benzene and benzene compounds, such as petrol.[19] The compound can interfere with cellular respiration, and kidney damage has been found in animals receiving severe exposure. It is excreted in its original form and also as variations of its own metabolite, hydroquinone.[6]
Benzoquinone compounds are a metabolite of paracetamol.[20]
Safety

1,4-Benzoquinone is able to stain skin dark brown, cause erythema (redness, rashes on skin) and lead on to localized tissue necrosis. It is particularly irritating to the eyes and respiratory system. Its ability to sublimate at commonly encountered temperatures allows for a greater airborne exposure risk than might be expected for a room-temperature solid. IARC has found insufficient evidence to comment on the compound's carcinogenicity, but has noted that it can easily pass into the bloodstream and that it showed activity in depressing bone marrow production in mice and can inhibit protease enzymes involved in cellular apoptosis.[6]
Related 1,4-benzoquinones
A variety of derivatives and analogues are known. Ubiquinone-1 is a naturally occurring 1,4-benzoquinone that is involved in respiration apparatus. The benzoquinone blattellaquinone is a sex pheromone in cockroaches.
|
Illustrative examples of quinones that are useful in industry and organic chemistry include:
- 1,4-Naphthoquinone, derived by oxidation of naphthalene with chromium trioxide.[21]
- 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ), a stronger oxidant and dehydrogenation agent than 1,4-benzoquinone.[22]
- Chloro-p-benzoquinone, (CAS no. [695-99-8])[23]
- Chloranil, 1,4-C6Cl4O2, a stronger oxidant and dehydrogenation agent than 1,4-benzoquinone.
See also
References
- 1 2 3 4 5 6 "NIOSH Pocket Guide to Chemical Hazards #0542". National Institute for Occupational Safety and Health (NIOSH).
- ↑ "Quinone". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- 1 2 Underwood, H. W. Jr.; Walsh, W. L. (1936). "Quinone". Org. Synth. 16: 73. doi:10.15227/orgsyn.002.0085.; Coll. Vol., 2, p. 553
- ↑ Gerhard Franz, Roger A. Sheldon "Oxidation" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2000 doi:10.1002/14356007.a18_261
- 1 2 Phillip M. Hudnall "Hydroquinone" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. 2005 Wiley-VCH, Weinheim. doi:10.1002/14356007.a13_499.
- 1 2 3 "1,4-BENZOQUINONE (para-QUINONE)" (pdf). IARC Monograph.
- ↑ Vliet, E. B. (1922). "Quinone". Org. Synth. 2: 85. doi:10.15227/orgsyn.016.0073.; Coll. Vol., 1, p. 482
- ↑ "Synthesis of para-Benzoquinone". erowid.org.
- ↑ US patent 4973720, "Process for the preparation of p-benzoquinone"
- 1 2 J.-M. Lü, S. V. Rosokha, I. S. Neretin and J. K. Kochi, "Quinones as Electron Acceptors. X-Ray Structures, Spectral (EPR, UV-vis) Characteristics and Electron-Transfer Reactivities of Their Reduced Anion Radicals as Separated vs. Contact Ion Pairs" Journal of the American Chemical Society 2006 128, 16708–19.doi:10.1021/ja066471o
- ↑ Cavallito, Chester J.; Soria, Albert E.; Hoppe, James O. (1950). "Amino- and Ammonium-alkylaminobenzoquinones as Curarimimetic Agents". Journal of the American Chemical Society 72 (6): 2661–2665. doi:10.1021/ja01162a088.
- ↑ Yang, T.-K.; Shen, C.-Y. (2004). "1,4-Benzoquinone". In L. Paquette. Encyclopedia of Reagents for Organic Synthesis. New York: J. Wiley & Sons. doi:10.1002/047084289X.rb033.
- ↑ Oda, M.; Kawase, T.; Okada, T.; Enomoto, T. (1996). "2-Cyclohexene-1,4-dione". Org. Synth. 73: 253. doi:10.15227/orgsyn.073.0253.; Coll. Vol., 9, p. 186
- ↑ Vliet, E. B. (1941). "Hydroquinone Triacetate". Organic Syntheses. 1: 317. doi:10.15227/orgsyn.004.0035.
- ↑ Knowles, M. B. (1952). "Process for production of 2,4,5-trihydroxyacetophenone" (PDF). Google Patents. Eastman Kodak Co. Retrieved 24 December 2014.
- ↑ McOmie, J. F. W.; Blatchly, J. M. "The Thiele-Winter Acetoxylation of Quinones". Organic Reactions. 19. doi:10.1002/0471264180.or019.03. ISBN 9780471196198.
- ↑ Thiele, J. (1898). "Ueber die Einwirkung von Essigsäure-anhydrid auf Chinon und auf Dibenzoylstyrol". Berichte der Deutschen Chemischen Gesellschaft. 31 (1): 1247–1249. doi:10.1002/cber.189803101226.
- ↑ Almeida, W. P.; Correia, C. R. D. (1999). "Stereoselective Total Synthesis and Enantioselective Formal Synthesis of the Antineoplastic Sesquiterpene Quinone Metachromin A" (pdf). Journal of the Brazilian Chemical Society. 10 (5): 401–414. doi:10.1590/S0103-50531999000500011.
- ↑ Lin, Y. S.; McKelvey, W.; Waidyanatha, S.; Rappaport, S. M. (2006). "Variability of Albumin Adducts of 1,4-Benzoquinone, a Toxic Metabolite of Benzene, in Human Volunteers". Biomarkers. 11 (1): 14–27. doi:10.1080/13547500500382975. PMID 16484134.
- ↑ Dahlin, D. C.; Miwa, G. T.; Lu, A. Y.; Nelson, S. D. (1984). "N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen". Proceedings of the National Academy of Sciences of the United States of America. 81 (5): 1327–1331. doi:10.1073/pnas.81.5.1327. PMC 344826
 . PMID 6424115.
. PMID 6424115. - ↑ Braude E. A.; Fawcett, J. S. (1953). "1,4-Naphthoquinone". Org. Synth. 33: 50. doi:10.15227/orgsyn.033.0050.; Coll. Vol., 4, p. 698
- ↑ Vogel, E.; Klug, W.; Breuer, A. (1974). "1,6-Methano[10]annulene". Org. Synth. 54: 11. doi:10.15227/orgsyn.054.0011.; Coll. Vol., 6, p. 731
- ↑ Harman, R. E. (1955). "Chloro-p-benzoquinone". Org. Synth. 35: 22. doi:10.15227/orgsyn.035.0022.; Coll. Vol., 4, p. 148
Appendix