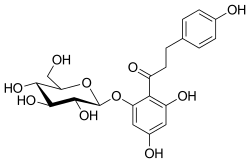
Phlorizin
 | |
 Haworth projection | |
| Names | |
|---|---|
| IUPAC name
1-[2,4-Вihydroxy-6-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]oxy-phenyl]-3-(4-hydroxyphenyl)propan-1-one | |
| Other names
Isosalipurposide | |
| Identifiers | |
| 60-81-1 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:8113 |
| ChEMBL | ChEMBL245067 |
| ChemSpider | 16498836 |
| ECHA InfoCard | 100.000.443 |
| 4757 | |
| PubChem | 6072 |
| UNII | CU9S17279X |
| |
| |
| Properties | |
| C21H24O10 | |
| Molar mass | 436.41 g·mol−1 |
| Appearance | White to yellow crystalline solid |
| Melting point | 106 to 109 °C (223 to 228 °F; 379 to 382 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Phlorizin, or phloretin-2'-β-D-glucopyranoside, also referred to as phloridzin and other names [1] is a glucoside of phloretin, a dihydrochalcone, a family of bicyclic flavonoids, which in turn is a subgroup in the diverse phenylpropanoid synthesis pathway in plants.
Occurrence
Phlorizin, or phloridzin is a naturally occurring flavonoid produced in some plants. It is found primarily in Malus (apple) species although trace amounts exist in other species. In Malus it is most abundant in vegetative tissues (leaves, bark, etc...) and seeds. Closely related species, such as pear (Pyrus communis), cherry and other fruit trees in the Rosaceae do not contain phloridzin.[2] Trace amounts have been reported in strawberry [3] and it is responsible for the petal color in Dianthus caryophyllus.[4] Phloridzin, a phytocemical belongs to a class of polyphenols. It may be present with other polyphenols such as quercetin, catechin, epicatechin, procynidins, rutin etc. These polyhydroxy compounds have been proved to be potent antioxidants.[5]
Properties
Phlorizin is a white to yellow crystalline solid with a melting point of 106–109 °C. It is of sweet taste and contains four molecules of water in the crystal. Above 200 °C, it decomposes. It is poorly soluble in ether and cold water, but soluble in ethanol and hot water. Upon prolonged exposure to aqueous solutions phlorizin hydrolyzes to phloretin and glucose.
Pharmacology
Phlorizin is a competitive inhibitor of SGLT1 and SGLT2 because it competes with D-glucose for binding to the carrier; this reduces renal glucose transport, lowering the amount of glucose in the blood.[6][7] Phlorizin was studied as a potential pharmaceutical treatment for type 2 diabetes, but has since been superseded by more selective and more promising synthetic analogs, such as canagliflozin and dapagliflozin.[8][9] Orally consumed phlorizin is nearly entirely converted into phloretin by hydrolytic enzymes in the small intestine.[10][11] Apart from conventional anti-diabetic activity, phlorizin is also studied for its antioxidant activity.[12]
References
- ↑ http://www.sigmaaldrich.com/catalog/product/aldrich/274313?lang=en
- ↑ Gosch, C.; Halbwirth, H.; Stich, K. (2010). "Phloridzin: biosynthesis, distribution and physiological relevance in plants". Phytochemistry. 71 (8): 838–843. doi:10.1016/j.phytochem.2010.03.003.
- ↑ Hilt, P.; Schieber, A.; Yildirim, C.; Arnold, G.; Klaiber, I.; Conrad, J.; Carle, R. (2003). "Detection of phloridzin in strawberries (Fragaria x ananassa Duch.) by HPLC-PDA-MS/MS and NMR spectroscopy". Journal of Agricultural and Food Chemistry. 51 (10): 2896–2899. doi:10.1021/jf021115k.
- ↑ Isosalipurposide on PubChem
- ↑ http://www.chemkind.com/chemicals-p_3685039_phloridzin.htm
- ↑ Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA (May 1987). "Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats". J Clin Invest. 79 (5): 1510–5. doi:10.1172/JCI112981. PMC 424427
 . PMID 3571496.
. PMID 3571496. - ↑ Tatoń, J; Piatkiewicz, P; Czech, A. (May–Jun 2010). "Molecular physiology of cellular glucose transport - a potential area for clinical studies in diabetes mellitus". Endokrynol Pol. 61 (3): 303–10. PMID 20602306.
- ↑ Chao, Edward C.; Henry, Robert R. (2010). "SGLT2 inhibition — a novel strategy for diabetes treatment". Nature Reviews Drug Discovery. 9 (7): 551–9. doi:10.1038/nrd3180. PMID 20508640.
- ↑ SGLT2 Inhibitors - UEndocrine.com
- ↑ Idris, I.; Donnelly, R. (2009). "Sodium-glucose co-transporter-2 inhibitors: An emerging new class of oral antidiabetic drug". Diabetes, Obesity and Metabolism. 11 (2): 79–88. doi:10.1111/j.1463-1326.2008.00982.x.
- ↑ Crespy, V.; Aprikian, O.; Morand, C.; Besson, C.; Manach, C.; Demigné, C.; Rémésy, C. (2001). "Bioavailability of phloretin and phloridzin in rats". The Journal of Nutrition. 131 (12): 3227–3230. PMID 11739871.
- ↑ http://www.chamberlins.com/ns/DisplayMonograph.asp?StoreID=2CB86C7B36BE4CFD914079104818C49B&DocID=bottomline-phlorizin