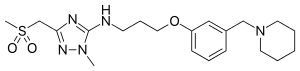
Sufotidine
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | None |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | 80343-63-1 |
| PubChem (CID) | 71763 |
| ChemSpider | 64802 |
| UNII | 56B0591Y76 |
| KEGG | D05939 |
| Chemical and physical data | |
| Formula | C20H31N5O3S |
| Molar mass | 421.556 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Sufotidine (INN,[1] USAN, codenamed AH25352) is a long-acting competitive H2 receptor antagonist which was under development as an antiulcerant by Glaxo (now GlaxoSmithKline).[2] It was planned to be a follow-up compound to ranitidine (Zantac).[3] When taken in doses of 600 mg twice daily it induced virtually 24-hour gastric anacidity[4] thus closely resembling the antisecretory effect of the proton pump inhibitor omeprazole.[5] Its development was terminated in 1989[6] from phase III clinical trials based on the appearance of carcinoid tumors in long-term toxicity testing in rodents.[7]
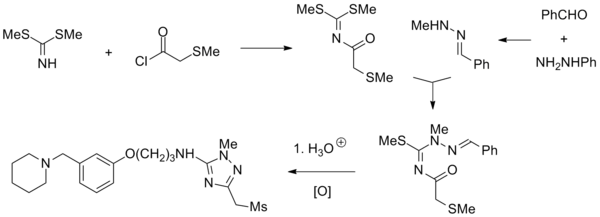
Synthesis
See also
- Lavoltidine (previously known as loxtidine) — a similar compound in which methylsulfone group is replaced with hydroxyl
References
- ↑ "International Nonproprietary Names for Pharmaceutical Substances. Supplement to WHO Chronicle, 1986, Vol. 40, No. 6. Recommended International Nonproprietary Names (Rec. INN): List 26" (PDF). World Health Organization. p. 9. Retrieved 12 January 2016.
- ↑ "Drug Profile: Sufotidine". AdisInsight. Springer International Publishing AG. Retrieved 12 January 2016.
- ↑ "Glaxo Plans to Follow Up Zantac with Long-acting Sufotidine; H2 Antagonist Is One of Seven Drugs Targeted for Worldwide Marketing Applications in 1988–90". Pharma & MedTech Business Intelligence. Informa Business Intelligence, Inc., an Informa Company. November 30, 1987. Retrieved 12 January 2016.
- ↑ Smith, JT; Pounder, RE (March 1990). "Sufotidine 600 mg bd Virtually Eliminates 24 hour Intragastric Acidity in Duodenal Ulcer Subjects". Gut. 31 (3): 291–3. doi:10.1136/gut.31.3.291. PMC 1378269
 . PMID 1969833.
. PMID 1969833. - ↑ Fiorucci, S; Santucci, L; Farroni, F; Pelli, MA; Morelli, A (October 1989). "Effect of Omeprazole on Gastroesophageal Reflux in Barrett's Esophagus". The American Journal of Gastroenterology. 84 (10): 1263–7. PMID 2801676.
- ↑ Ganellin, C.R.; Triggle, D.J., eds. (1999). Dictionary of Pharmacological Agents (1st ed.). London: Chapman & Hall. ISBN 9780412466304.
- ↑ Lawton, G.; Witty, D.R., eds. (2011). Progress in Medicinal Chemistry. Vol. 50 (1st ed.). London: Academic. p. 36. ISBN 978-0-12-381290-2.
This article is issued from Wikipedia - version of the 7/31/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.