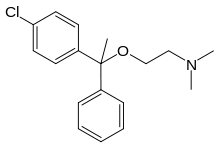
Chlorphenoxamine
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral, topical |
| ATC code | D04AA34 (WHO) R06AA06 (WHO) |
| Pharmacokinetic data | |
| Bioavailability | Well absorbed |
| Metabolism | Likely hepatic |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
77-38-3 |
| PubChem (CID) | 6475 |
| DrugBank |
DB09007 |
| ChemSpider |
6230 |
| UNII |
3UVD77BP8R |
| KEGG |
D07198 |
| ChEMBL |
CHEMBL2110774 |
| ECHA InfoCard | 100.115.538 |
| Chemical and physical data | |
| Formula | C18H22ClNO |
| Molar mass | 303.826 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Chlorphenoxamine (Phenoxene) is an antihistamine and anticholinergic used as an antipruritic[1] and antiparkinsonian[2] agent. It is an analog of diphenhydramine.[3]
References
- ↑ Bazex, A.; Dupre, A.; Christol, B. (1963). "Trial treatment of urticaria with chlorphenoxamine". Clinique (Paris, France). 58: 447–450. PMID 13967113.
- ↑ Uldall, P. R.; Walton, J. N.; Newell, D. J. (1961). "Chlorphenoxamine hydrochloride in parkinsonism. A controlled trial". British Medical Journal. 1 (5240): 1649–1652. doi:10.1136/bmj.1.5240.1649. PMC 1954253
 . PMID 13779077.
. PMID 13779077. - ↑ H. Arnold, N. Brock, E. Kuhas, and D. Lorenz, Arzneimittel Forsch., 4, 189 (1954).
| Antihistamines for topical use | |
|---|---|
| Anesthetics for topical use | |
| Others |
|
| Dopaminergics |
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anticholinergics | |||||||||||||||||||
| Others | |||||||||||||||||||
| |||||||||||||||||||
| Aminoalkyl ethers |
|
|---|---|
| Substituted alkylamines | |
| Substituted ethylenediamines |
|
| Phenothiazine derivatives | |
| Piperazine derivatives | |
| Others for systemic use |
|
| For topical use | |
| Receptor (ligands) |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (inhibitors) |
| ||||||||||||||
| Enzyme (inhibitors) |
| ||||||||||||||
| Others |
| ||||||||||||||
This article is issued from Wikipedia - version of the 4/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.