Verteporfin
 | |
| Clinical data | |
|---|---|
| Trade names | Visudyne |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607060 |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | S01LA01 (WHO) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
129497-78-5 |
| PubChem (CID) | 5362420 |
| DrugBank |
DB00460 |
| ChemSpider |
21106402 |
| UNII |
0X9PA28K43 |
| KEGG |
D01162 |
| ChEBI |
CHEBI:60775 |
| ChEMBL |
CHEMBL2218885 |
| Chemical and physical data | |
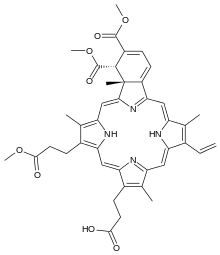
| Formula | C41H42N4O8 |
| Molar mass | 718.794 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Verteporfin (trade name Visudyne), a benzoporphyrin derivative, is a medication used as a photosensitizer for photodynamic therapy to eliminate the abnormal blood vessels in the eye associated with conditions such as the wet form of macular degeneration. Verteporfin accumulates in these abnormal blood vessels and, when stimulated by nonthermal red light with a wavelength of 689 nm[1] in the presence of oxygen, produces highly reactive short-lived singlet oxygen and other reactive oxygen radicals, resulting in local damage to the endothelium and blockage of the vessels.[2][3]
Verteporfin is also used off-label for the treatment of central serous retinopathy.[4]
Administration
Verteporfin is given intravenously, 15 minutes before laser treatment.[2]
Contraindications
Side effects
Most common side effects are blurred vision, headache, and local effects at the injection site. Also, photosensitivity; it is advised to avoid exposure to sunlight and unscreened lighting until 48 hours after the injection of verteporfin.[2]
Interactions
Verteporfin is known to interact with the herbal remedy feverfew (Tanacetum parthenium), the latter of which seems to act as an antagonist to verteporfin for unknown reasons. Taking the two substances simultaneously is inadvisable.[5]
Verteporfin has no influence on the liver enzyme CYP3A4, which metabolises many pharmaceutical drugs.[2]
References
- ↑ "Visudyne package insert" (PDF).
- 1 2 3 4 5 Verteporfin Monograph
- ↑ Scott, L. J.; Goa, K. L. (2000). "Verteporfin". Drugs & aging. 16 (2): 139–146; discussion 146–8. doi:10.2165/00002512-200016020-00005. PMID 10755329.
- ↑ Adelman, R.; Adelman, R. A. (2013). "Profile of verteporfin and its potential for the treatment of central serous chorioretinopathy". Clinical Ophthalmology. 7: 1867–1875. doi:10.2147/OPTH.S32177. PMC 3788817
 . PMID 24092965.
. PMID 24092965. - ↑ "Feverfew and Verteporfin Interactions". Retrieved 14 April 2015.