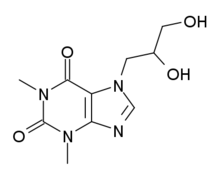
Dyphylline
 | |
| Clinical data | |
|---|---|
| Trade names | Lufyllin |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a682494 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | R03DA01 (WHO) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Synonyms | 7-(2,3-dihydroxy-propyl)theophylline |
| CAS Number |
479-18-5 |
| PubChem (CID) | 3182 |
| IUPHAR/BPS | 7070 |
| DrugBank |
DB00651 |
| ChemSpider |
3070 |
| UNII |
263T0E9RR9 |
| KEGG |
D00691 |
| ChEBI |
CHEBI:4728 |
| ChEMBL |
CHEMBL1752 |
| ECHA InfoCard | 100.006.843 |
| Chemical and physical data | |
| Formula | C10H14N4O4 |
| Molar mass | 254.24 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Dyphylline (USAN) (trade names Dilor, Lufyllin), also known as diprophylline (INN), is a xanthine derivative with bronchodilator and vasodilator effects. It is used in the treatment of respiratory disorders like asthma, cardiac dyspnea, and bronchitis. It acts as an adenosine receptor antagonist and phosphodiesterase inhibitor.[1][2]
See also
References
- ↑ Schwabe U, Ukena D, Lohse MJ (September 1985). "Xanthine derivatives as antagonists at A1 and A2 adenosine receptors". Naunyn-Schmiedeberg's Archives of Pharmacology. 330 (3): 212–21. doi:10.1007/bf00572436. PMID 2997628.
- ↑ Iancu L, Shneur A, Cohen H (1979). "Trials with xanthine derivatives in systemic treatment of psoriasis". Dermatologica. 159 (1): 55–61. doi:10.1159/000250562. PMID 225216.
| Adrenergics, inhalants |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucocorticoids | |||||||||||||||
| Anticholinergics/ muscarinic antagonist | |||||||||||||||
| Mast cell stabilizers | |||||||||||||||
| Xanthines | |||||||||||||||
| Eicosanoid inhibition |
| ||||||||||||||
| Others/unknown | |||||||||||||||
| Combination products | |||||||||||||||
| |||||||||||||||
| Receptor (ligands) |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (blockers) |
| ||||||||||||||||
| Enzyme (inhibitors) |
| ||||||||||||||||
| Others | |||||||||||||||||
This article is issued from Wikipedia - version of the 9/14/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.