Glutathione disulfide
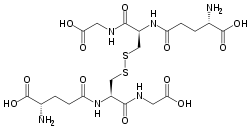 | |
| Names | |
|---|---|
| IUPAC name
(2S)-2-amino-5-[[(2R)-3-[(2R)-2-[[(4S)-4-amino-5-hydroxy-5-oxopentanoyl]amino]-3-(carboxymethylamino)-3-oxopropyl]disulfanyl-1-
(carboxymethylamino)-1-oxopropan-2-yl]amino]-5-oxopentanoic acid | |
| Identifiers | |
| 27025-41-8 | |
| 3D model (Jmol) | Interactive image |
| Abbreviations | GSSG |
| ChEMBL | ChEMBL1372 |
| ChemSpider | 58835 |
| ECHA InfoCard | 100.043.777 |
| 6835 | |
| PubChem | 65359 11215652 |
| |
| |
| Properties | |
| C20H32N6O12S2 | |
| Molar mass | 612.631 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Glutathione disulfide (GSSG) is a disulfide derived from two glutathione molecules.[1]
In living cells, glutathione disulfide is reduced into two molecules of glutathione with reducing equivalents from the coenzyme NADPH. This reaction is catalyzed by the enzyme glutathione reductase.[2] Antioxidant enzymes, such as glutathione peroxidases and peroxiredoxins, generate glutathione disulfide during the reduction of peroxides such as hydrogen peroxide (H2O2) and organic hydroperoxides (ROOH):[3]
- 2 GSH + ROOH → GSSG + ROH + H2O
Other enzymes, such as glutaredoxins, generate glutathione disulfide through thiol-disulfide exchange with protein disulfide bonds or other low molecular mass compounds, such as coenzyme A disulfide or dehydroascorbic acid.[4]
- 2 GSH + R-S-S-R → GSSG + 2 RSH
Neuromodulator
GSSG, along with glutathione and S-nitrosoglutathione (GSNO), have been found to bind to the glutamate recognition site of the NMDA and AMPA receptors (via their γ-glutamyl moieties), and may be endogenous neuromodulators.[5][6] At millimolar concentrations, they may also modulate the redox state of the NMDA receptor complex.[6]
See also
- Glutathione-ascorbate cycle
- NOV-002
- NOV-205
- Antioxidant
References
- ↑ Meister A, Anderson M (1983). "Glutathione". Annu Rev Biochem. 52: 711–60. doi:10.1146/annurev.bi.52.070183.003431. PMID 6137189.
- ↑ Deneke SM, Fanburg BL (October 1989). "Regulation of cellular glutathione". Am. J. Physiol. 257 (4 Pt 1): L163–73. PMID 2572174.
- ↑ Meister A (1988). "Glutathione metabolism and its selective modification" (PDF). J Biol Chem. 263 (33): 17205–8. PMID 3053703.
- ↑ Holmgren A, Johansson C, Berndt C, Lönn ME, Hudemann C, Lillig CH (December 2005). "Thiol redox control via thioredoxin and glutaredoxin systems". Biochem. Soc. Trans. 33 (Pt 6): 1375–7. doi:10.1042/BST20051375. PMID 16246122.
- ↑ Steullet, P.; Neijt, H.C.; Cuénod, M.; Do, K.Q. (2006). "Synaptic plasticity impairment and hypofunction of NMDA receptors induced by glutathione deficit: Relevance to schizophrenia". Neuroscience. 137 (3): 807–819. doi:10.1016/j.neuroscience.2005.10.014. ISSN 0306-4522.
- 1 2 Varga, V.; Jenei, Zs.; Janáky, R.; Saransaari, P.; Oja, S. S. (1997). "Glutathione Is an Endogenous Ligand of Rat Brain N-Methyl-D-Aspartate (NMDA) and 2-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionate (AMPA) Receptors". Neurochemical Research. 22 (9): 1165–1171. doi:10.1023/A:1027377605054. ISSN 0364-3190.