Porin (protein)
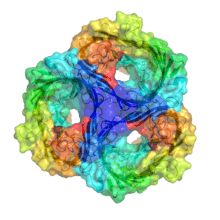

| Gram-negative porin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
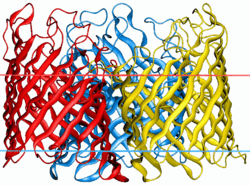 | |||||||||
| Identifiers | |||||||||
| Symbol | Porin_1 | ||||||||
| Pfam | PF00267 | ||||||||
| Pfam clan | CL0193 | ||||||||
| InterPro | IPR001702 | ||||||||
| PROSITE | PDOC00498 | ||||||||
| SCOP | 1mpf | ||||||||
| SUPERFAMILY | 1mpf | ||||||||
| TCDB | 1.B.1 | ||||||||
| OPM superfamily | 31 | ||||||||
| OPM protein | 1pho | ||||||||
| CDD | cd01345 | ||||||||
| |||||||||
Porins are beta barrel proteins that cross a cellular membrane and act as a pore, through which molecules can diffuse.[1] Unlike other membrane transport proteins, porins are large enough to allow passive diffusion, i.e., they act as channels that are specific to different types of molecules. They are present in the outer membrane of gram-negative bacteria and some gram-positive bacteria of the group Mycolata (mycolic acid-containing actinomycetes), the mitochondria, and the chloroplast.
Structure
Porins are composed of β strands, which are, in general, linked together by beta turns on the cytoplasmic side and long loops of amino acids on the other. The β strands lie in an antiparallel fashion and form a cylindrical tube, called a β barrel.[2] The amino acid composition of the porin β strands are unique in that polar and nonpolar residues alternate along them. This means that the nonpolar residues face outward so as to interact with the nonpolar lipids of outer membrane, whereas the polar residues face inwards into the center of the beta barrel to create the aqueous channel.
The porin channel is partially blocked by a loop, called the eyelet, which projects into the cavity. In general, it is found between strands 5 and 6 of each barrel, and it defines the size of solute that can traverse the channel. It is lined almost exclusively with charged amino acids arranged on opposite sides of the channel, creating a transversal electric field across the pore. The eyelet has a local surplus of negative charges from four glutamic acid and seven aspartic acid residues (in contrast to one histidine, two lysine and three arginine residues) is partially compensated for by two bound calcium atoms, and this asymmetric arrangement of molecules is thought to have an influence in the selection of molecules that can pass through the channel.[3]
Cellular roles
To transport medium-sized or charged molecules across, the molecules move through a porin, a water-filled channel or pore.[4][5]
Porins typically control the diffusion of small metabolites like sugars, ions, and amino acids.
In gram-negative bacteria, the inner membrane is the major permeability barrier, whereas the outer membrane contains porins, which render it largely permeable to molecules less than about 1500 daltons.
Porins are chemically selective – transporting only one group of molecules – or may be specific for one molecule. Beta-lactam and fluoroquinolone antibiotics must pass through porins to reach their targets in gram negative bacteria. Bacteria can develop resistance to these antibiotics by mutating the gene that encodes the porin – the antibiotics are then excluded from passing through the outer membrane.
The term "nucleoporin" refers to completely unrelated proteins facilitating transport through nuclear pores in the nuclear envelope.
Discoverer
The discovery of porins has been attributed to Hiroshi Nikaido, nicknamed "the porinologist."[6]
Porin Superfamilies
Porin superfamily I includes 47 families of porins with a range of numbers of trans-membrane β-strands (β-TMS). These include the GBP, SP and RPP porin families. The following table lists the families behaved to belong to Porin Superfamilies (PSF) I - V. While PSF I includes 47 families, PSF II-V each contain only 2 families. While PSF I derives members from gram-negative bacteria primarily one family of eukaryotic mitochondrial porins, PSF II and V porins are derived from Actinobacteria. PSF III and V are derived from eukaryotic organelle. See TCDB for more details.[7][8]
Porin Superfamily I
1.B.1 - The General Bacterial Porin (GBP) Family
1.B.2 - The Chlamydial Porin (CP) Family
1.B.3 - The Sugar Porin (SP) Family
1.B.4 - The Brucella-Rhizobium Porin (BRP) Family
1.B.5 - The Pseudomonas OprP Porin (POP) Family
1.B.6 - The OmpA-OmpF Porin (OOP) Family
1.B.7 - The Rhodobacter PorCa Porin (RPP) Family
1.B.8 - The Mitochondrial and Plastid Porin (MPP) Family
1.B.9 - The FadL Outer Membrane Protein (FadL) Family
1.B.10 - The Nucleoside-specific Channel-forming Outer Membrane Porin (Tsx) Family
1.B.11 - The Outer Membrane Fimbrial Usher Porin (FUP) Family
1.B.12 - The Autotransporter-1 (AT-1) Family
1.B.13 - The Alginate Export Porin (AEP) Family
1.B.14 - The Outer Membrane Receptor (OMR) Family
1.B.15 - The Raffinose Porin (RafY) Family
1.B.16 - The Short Chain Amide and Urea Porin (SAP) Family
1.B.17 - The Outer Membrane Factor (OMF) Family
1.B.18 - The Outer Membrane Auxiliary (OMA) Protein Family
1.B.19 - The Glucose-selective OprB Porin (OprB) Family
1.B.20 - The Two-Partner Secretion (TPS) Family
1.B.21 - The OmpG Porin (OmpG) Family
1.B.22 - The Outer Bacterial Membrane Secretin (Secretin) Family
1.B.23 - The Cyanobacterial Porin (CBP) Family
1.B.25 - The Outer Membrane Porin (Opr) Family
1.B.26 - The Cyclodextrin Porin (CDP) Family
1.B.31 - The Campylobacter jejuni Major Outer Membrane Porin (MomP) Family
1.B.32 - The Fusobacterial Outer Membrane Porin (FomP) Family
1.B.33 - The Outer Membrane Protein Insertion Porin (Bam Complex) (OmpIP) Family
1.B.35 - The Oligogalacturonate-specific Porin (KdgM) Family
1.B.39 - The Bacterial Porin, OmpW (OmpW) Family
1.B.42 - The Outer Membrane Lipopolysaccharide Export Porin (LPS-EP) Family
1.B.43 - The Coxiella Porin P1 (CPP1) Family
1.B.44 - The Probable Protein Translocating Porphyromonas gingivalis Porin (PorT) Family
1.B.49 - The Anaplasma P44 (A-P44) Porin Family
1.B.54 - The Intimin/Invasin (Int/Inv) or Autotransporter-3 (AT-3) Family
1.B.55 - The Poly Acetyl Glucosamine Porin (PgaA) Family
1.B.57 - The Legionella Major-Outer Membrane Protein (LM-OMP) Family
1.B.60 - The Omp50 Porin (Omp50 Porin) Family
1.B.61 - The Delta-Proteobacterial Porin (Delta-Porin) Family
1.B.62 - The Putative Bacterial Porin (PBP) Family
1.B.66 - The Putative Beta-Barrel Porin-2 (BBP2) Family
1.B.67 - The Putative Beta Barrel Porin-4 (BBP4) Family
1.B.68 - The Putative Beta Barrel Porin-5 (BBP5) Superfamily
1.B.70 - The Outer Membrane Channel (OMC) Family
1.B.71 - The Proteobacterial/Verrucomicrobial Porin (PVP) Family
1.B.72 - The Protochlamydial Outer Membrane Porin (PomS/T) Family
1.B.73 - The Capsule Biogenesis/Assembly (CBA) Family
1.B.78 - The DUF3374 Electron Transport-associated Porin (ETPorin) Family
Porin Superfamily II (MspA Superfamily)
1.B.24 - The Mycobacterial Porin (MBP) Family
1.B.58 - Nocardial Hetero-oligomeric Cell Wall Channel (NfpA/B) Family
Porin Superfamily III
1.B.28 - The Plastid Outer Envelope Porin of 24 kDa (OEP24) Family
1.B.47 - The Plastid Outer Envelope Porin of 37 kDa (OEP37) Family
Porin Superfamily IV (Tim17/OEP16/PxMPL (TOP) Superfamily)
This superfamily includes protein that comprise pores in multicomponent protein translocases as follows: 3.A.8 - [Tim17 (P39515) Tim22 (Q12328) Tim23 (P32897)]; 1.B.69 - [PXMP4 (Q9Y6I8) PMP24 (A2R8R0)]; 3.D.9 - [NDH 21.3 kDa component (P25710)]
1.B.30 - The Plastid Outer Envelope Porin of 16 kDa (OEP16) Family
1.B.69 - The Peroxysomal Membrane Porin 4 (PxMP4) Family
3.A.8 - The Mitochondrial Protein Translocase (MPT) Family
Porin Superfamily V (Corynebacterial PorA/PorH Superfamily)
1.B.34 - The Corynebacterial Porin A (PorA) Family 1.B.59 - The Outer Membrane Porin, PorH (PorH) Family
See also
References
- ↑ Porins at the US National Library of Medicine Medical Subject Headings (MeSH)
- ↑ Schirmer T (1998). "General and specific porins from bacterial outer membranes". J. Struct. Biol. 121 (2): 101–9. doi:10.1006/jsbi.1997.3946. PMID 9615433.
- ↑ Branden and Tooze, Introduction to Protein Structure, second edition
- ↑ "Current Protein and Peptide Science". doi:10.2174/138920312804871120. Retrieved 2016-10-14.
- ↑ "Structure and Functional Mechanism of Potins". Physiological Reviews. 76.
- ↑ Klebba PE (December 2005). "The porinologist". J. Bacteriol. 187 (24): 8232–6. doi:10.1128/JB.187.24.8232-8236.2005. PMC 1317029
 . PMID 16321927.
. PMID 16321927. - ↑ Niederweis, Michael (2003-09-01). "Mycobacterial porins--new channel proteins in unique outer membranes". Molecular Microbiology. 49 (5): 1167–1177. doi:10.1046/j.1365-2958.2003.03662.x. ISSN 0950-382X. PMID 12940978.
- ↑ Rath, Parthasarathi; Saurel, Olivier; Tropis, Maryelle; Daffé, Mamadou; Demange, Pascal; Milon, Alain (2013-11-15). "NMR localization of the O-mycoloylation on PorH, a channel forming peptide from Corynebacterium glutamicum". FEBS Letters. 587 (22): 3687–3691. doi:10.1016/j.febslet.2013.09.032. ISSN 1873-3468. PMID 24100136.
External links
- Overview at University of Hamburg
- UMich Orientation of Proteins in Membranes families/superfamily-181 - OmpG porin
- UMich Orientation of Proteins in Membranes families/superfamily-31 - Trimeric porins
- UMich Orientation of Proteins in Membranes families/superfamily-32 - Sugar porins