SK channel
| Calcium-activated SK potassium channel | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
SK Channel | |||||||||
| Identifiers | |||||||||
| Symbol | SK_channel | ||||||||
| Pfam | PF03530 | ||||||||
| InterPro | IPR015449 | ||||||||
| |||||||||
SK channels (Small conductance calcium-activated potassium channels) are a subfamily of Ca2+-activated K+ channels.[1] They are so called because of their small single channel conductance in the order of 10 pS.[2] SK channels are a type of ion channel allowing potassium cations to cross the cell membrane and are activated (opened) by an increase in the concentration of intracellular calcium through N-type calcium channels. Their activation limits the firing frequency of action potentials and is important for regulating afterhyperpolarization in the neurons of the central nervous system as well as many other types of electrically excitable cells. This is accomplished through the hyperpolarizing leak of positively charged potassium ions along their concentration gradient into the extracellular space. This hyperpolarization causes the membrane potential to become more negative.[3] SK channels are thought to be involved in synaptic plasticity and therefore play important roles in learning and memory.[4]
Function
SK channels are expressed throughout the central nervous system. They are highly conserved in mammals as well as in other organisms such as Drosophila melanogaster and Caenorhabditis elegans.[5] SK channels are specifically involved in the medium afterhyperpolarizing potential (mAHP). They affect both the intrinsic excitability of neurons and synaptic transmission. They are also involved in calcium signaling.[6] SK channels control action potential discharge frequency in hippocampal neurons, midbrain dopaminergic neurons, dorsal vagal neurons, sympathetic neurons, nucleus reticularis thalmic neurons, inferior olive neurons, spinal and hypoglossal motoneurons, mitrial cells in the olfactory bulb, and cortical neurons.[3]
Structure
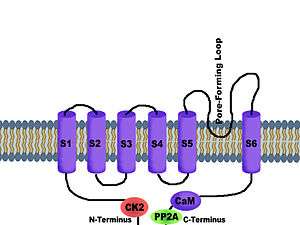
SK potassium channels share the same basic architecture with Shaker-like voltage-gated potassium channels.[7] Four subunits associate to form a tetramer. Each of the subunits has six transmembrane hydrophobic alpha helical domains (S1-S6). A loop between S5 and S6—called the P-loop—provides the pore-forming region that always faces the center of the channel.[8] Each of the subunits has six hydrophobic alpha helical domains that insert into the cell membrane. A loop between the fifth and sixth transmembrane domains forms the potassium ion selectivity filter. SK channels may assemble as homotetrameric channels or as heterotetrameric channels, consisting of more than one SK channel subtype. In addition, SK potassium channels are tightly associated with the protein calmodulin, which accounts for the calcium sensitivity of these channels.[7][9] Calmodulin participates as a subunit of the channel itself, bound to the cytoplasmic C-terminus region of the peptide called the calmodulin binding domain (CaMBD).[10]
Additional association of the phosphorylating kinase CK2 and dephosphorylating phosphatase PP2A on the cytoplasmic face of the protein allow for enriched Ca2+-sensitivity—and thus—kinetics modulation.[11] CK2 serves to phosphorylate the SKCa-bound CaM at the T80 residue, rather than the channel helices themselves, to reduce calcium sensitivity. This may only be accomplished when the channel pore is closed. PP2A dephosphorylates this residue upon CK2 inhibition.[10] The selectivity filter of all SK channel subtypes—whether SK1, SK2, SK3, or SK4—is highly conserved and reflects the selectivity seen in any potassium channel, a GYGD amino acid residue sequence on the pore-forming loop.[12] These channels are considered to be voltage-independent, as they possess only two of seven positively charged amino acid residues that are typically seen in a prototypical voltage-gated potassium channel.[8]
Classification
The SK channel family contains 4 members - SK1, SK2, SK3, and SK4. SK4 is often referred to as IK (Intermediate conductance) due to its higher conductance 20 - 80 pS.[13]
| Channel | Gene | Aliases | Associated subunits |
| SK1 | KCNN1 | Kca2.1 | calmodulin, PP2A, CK2 |
| SK2 | KCNN2 | Kca2.2 | calmodulin, PP2A, CK2 |
| SK3 | KCNN3 | Kca2.3 | calmodulin, PP2A, CK2 |
| SK4 | KCNN4 | Kca3.1 | calmodulin, PP2A, CK2 |
Gating Mechanism
The SK channel gating mechanism is controlled by intracellular calcium levels.[5] Calcium enters the cell via voltage activated calcium channels as well as through NMDA receptors.[3] Calcium does not directly bind to the SK channel. Calcium binds to the protein calmodulin (CaM). When bound to calcium, CaM binds to the CaM-binding domain on the intracellular subunit of the SK channel. When each of the four CaM-binding domain subunits is bound to calmodulin, the SK channel changes conformation. This transitions the channel from a tetramer of monomers to a folded dimer of dimers, which results in rotation of the CaM-binding domains. This rotation causes the mechanical opening of the channel gate.[5] The time constant of SK channel activation is approximately 5 ms. When calcium levels are depleted, the time constant for channel deactivation ranges from 15-60 ms.[14]
Blockers
All SK channels can be pharmacologically blocked by quaternary ammonium salts of a plant-derived neurotoxin bicuculline.[15] In addition, SK channels (SK1-SK3) but not SK4 (IK) are sensitive to blockade by the bee toxin apamin,[16] and the scorpion venoms tamapin and charybdotoxin (ChTx), all via competitive antagonism for access to the mouth of the pore formation.[17] All known blockers compete for roughly the same binding site, the pore, in all subtypes. This provides a physical blockage to the channel pore.[18] Since all blockers are universal to all three types of SK channels, there is an incredibly narrow therapeutic window that does not allow for blocking of a specific SK channel subtype.[11] Quaternary ammonium salts like bicuculline and tetraethylammonium (TEA) enter the pore via the selectivity filter by acting as a potassium mimic in the dehydration step of pore permeation.[18]
The following molecules are other toxins and organic compounds that also inhibit all three small SK channel subtypes to any (even minimal) degree:[11]
- Dequalinium
- d-Tubocurarine
- UCl-1684
- UCL-1848
- Cyproheptadine
- Fluoxetine, the active ingredient in Prozac
- NS8593
- Scyllatoxin (Leiurotoxin-I)
- Lei-Dab7
- N-methyl-laudanosine
- N-Me-bicuculline
- Pancuronium
- Atracurium
- 1-ethyl-1H-benzo[d]imidazol-2(3H)-on
- 6,7-dichloro-3-(hydroxyimino)indolin-2-one
- N-cyclohexyl-2-(3,5-dimethyl-1H-pyrazol-1-yl)-6-methylpyrimidin-4-amine
- (R)-N-(1,2,3,4-tetrahydronaphthalen-1-yl)-1H-benzo[d]imidazol-2-amine
Modulators
Allosteric modulators of small SK channels work by changing the apparent calcium sensitivity of the channels. Examples include:
- Non-selective positive modulators of SK channels: EBIO (1-Ethyl-2-BenzimIdazolinOne),[19] NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime)[20]
- SK-2 and SK-3 selective positive modulators : CyPPA (NS6277; Cyclohexyl-(2-(3,5-dimethyl-Pyrazol-1-yl)-6-methyl-Pyrimidin-4-yl)-Amine)[21]

Synaptic plasticity and long term potentiation
In dendritic spines, SK channels are directly coupled to NMDA receptors. In addition to being activated by calcium flow through voltage-gated calcium channels, SK channels can be activated by calcium flowing through NMDA receptors, which occurs after depolarization of the postsynaptic membrane.[10] Experiments using apamin have shown that specifically blocking SK channels can increase learning and long-term potentiation. In addition, brain-derived neurotrophic factor (BDNF) causes the down-regulation of SK channels, which facilitates long-term potentiation. Increasing SK channel activity has the opposite effect and serves to impair learning.[5] An increase in SK channel activity that occurs over time may be related to decreases in plasticity and memory that is seen with aging.[22]
Role in Parkinson’s Disease
The dysfunction of potassium channels, including SK channels, is thought to play a role in the pathogenesis of Parkinson's disease (PD), a progressive neurodegenerative disorder.
SK channel blockers control the firing rate (the number of action potentials produced by a neuron in a given time) and the firing pattern (the way action potentials are allocated throughout time) through their production of m-AHP. SK channel activators decrease the firing rate and neuron sensitivity to excitatory stimuli, whereas SK channel blockers increase the firing rate and sensitivity to excitatory stimuli. This has important implications as to the function of dopaminergic neurons.[23] For example, the amount of dopamine released by midbrain dopaminergic neurons is much higher when the frequency of firing increases than when they fire at a constant rate.
SK channels are widely expressed in midbrain dopaminergic neurons. Multiple pharmacological techniques have been used to adjust SK affinity for calcium ions, thereby modulating the excitability of substantia nigra dopaminergic neurons. Blockage of SK channels in vivo increases the firing rate of substantia nigra cells, which increases the amount of dopamine released from the synaptic terminals.[23] When a large amount of dopamine accumulates in the cytosol, cell damage is induced due to the build-up of free radicals and damage to mitochondria. In addition, techniques have been used to modulate SK channels in order to alter the dopamine phenotype of neurons. After the loss of TH+ (tyrosine hydroxylase-positive) substantia nigra compacta (SNc) neurons due to Parkinson’s-induced neurodegeneration, the number of these neurons can partially recover via a cell phenotype “shift” from TH- (tyrosine hydroxylase-negative) to TH+. The number of TH+ neurons can be altered by SK channel modulation; to be specific, the infusion of SK agonists into substantia nigra increases the number of TH+ neurons, whereas the infusion of SK anatagonist decreases the number of TH+ neurons. The reason for this relationship between SK channels and TH expression may be due to neuroprotection against dopamine toxicity.[23]
Based on the two opposing roles of SK channels in the pathogenesis of PD, two contradictory methods have been suggested as therapeutic options for the improvement of PD symptoms: Inhibition of SK channels
- Inhibition of SK channels, to be specific the blockage of SK3 channels, increases the frequency of firing in dopaminergic neurons, thereby increasing the release of dopamine. It is, therefore, thought that the application of SK3 channels blockers in PD patients may alleviate short-term motor symptoms.
- However, inhibition also results in a decreased number of TH+ substantia nigra compacta (SNc) neurons in the cell, which results in a decrease in dopamine synthesis over the long term.
Facilitation of SK channels
- Enhancing the function of SK channels increases the number of TH+ substantia nigra compacta (SNc) neurons in the cell, thereby maintaining dopamine synthesis over the long term.
- However, the facilitation of SK channels decreases the firing frequency in dopaminergic neurons over the short term.
References
- ↑ Bond CT, Maylie J, Adelman JP (1999). "Small-conductance calcium-activated potassium channels". Ann. N. Y. Acad. Sci. 868 (1): 370–8. doi:10.1111/j.1749-6632.1999.tb11298.x. PMID 10414306.
- ↑ Köhler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP (1996). "Small-conductance, calcium-activated potassium channels from mammalian brain". Science. 273 (5282): 1709–14. doi:10.1126/science.273.5282.1709. PMID 8781233.
- 1 2 3 Faber ES, Sah P (2007). "Functions of SK channels in central neurons". Clin. Exp. Pharmacol. Physiol. 34 (10): 1077–83. doi:10.1111/j.1440-1681.2007.04725.x. PMID 17714097.
- ↑ Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, Tzounopoulos T (2002). "Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding". J. Neurosci. 22 (23): 10163–71. PMID 12451117.
- 1 2 3 4 Adelman JP, Maylie J, Sah P (2012). "Small-conductance Ca2+-activated K+ channels: form and function". Annu. Rev. Physiol. 74: 245–69. doi:10.1146/annurev-physiol-020911-153336. PMID 21942705.
- ↑ Dolga AM, Terpolilli N, Kepura F, Nijholt IM, Knaus HG, D'Orsi B, Prehn JH, Eisel UL, Plant T, Plesnila N, Culmsee C (April 2011). "KCa2 channels activation prevents [Ca2+]i deregulation and reduces neuronal death following glutamate toxicity and cerebral ischemia.". Cell Death Dis. 2 (e147): e147. doi:10.1038/cddis.2011.30. PMC 3122061
 . PMID 21509037.
. PMID 21509037. - 1 2 Maylie J, Bond CT, Herson PS, Lee WS, Adelman JP (2004). "Small conductance Ca2+-activated K+ channels and calmodulin". J. Physiol. (Lond.). 554 (Pt 2): 255–61. doi:10.1113/jphysiol.2003.049072. PMC 1664776
 . PMID 14500775.
. PMID 14500775. - 1 2 Stocker M (October 2004). "Ca2+-activated K+ channels: molecular determinants and function of the SK family". Nature Reviews Neuroscience. 5 (10): 758–70. doi:10.1038/nrn1516. PMID 15378036.
- ↑ Schumacher MA, Rivard AF, Bächinger HP, Adelman JP (2001). "Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin". Nature. 410 (6832): 1120–4. doi:10.1038/35074145. PMID 11323678.
- 1 2 3 Luján R, Maylie J, Adelman JP (July 2009). "New sites of action for GIRK and SK channels". Nature Reviews Neuroscience. 10 (7): 475–80. doi:10.1038/nrn2668. PMID 19543219.
- 1 2 3 Weatherall KL, Goodchild SJ, Jane DE, Marrion NV (July 2010). "Small conductance calcium-activated potassium channels: from structure to function". Prog. Neurobiol. 91 (3): 242–55. doi:10.1016/j.pneurobio.2010.03.002. PMID 20359520.
- ↑ Bernèche S, Roux B (April 2005). "A gate in the selectivity filter of potassium channels". Structure. 13 (4): 591–600. doi:10.1016/j.str.2004.12.019. PMID 15837197.
- ↑ Vergara C, Latorre R, Marrion NV, Adelman JP (1998). "Calcium-activated potassium channels". Current Opinion in Neurobiology. 8 (Pt 3): 321–9. doi:10.1016/S0959-4388(98)80056-1. PMID 9687354.
- ↑ Berkefeld H, Fakler B, Schulte U (October 2010). "Ca2+-activated K+ channels: from protein complexes to function". Physiol. Rev. 90 (4): 1437–59. doi:10.1152/physrev.00049.2009. PMID 20959620.
- ↑ Khawaled R, Bruening-Wright A, Adelman JP, Maylie J (1999). "Bicuculline block of small-conductance calcium-activated potassium channels". Pflugers Arch. 438 (3): 314–21. doi:10.1007/s004240050915. PMID 10398861.
- ↑ Blatz AL, Magleby KL (1986). "Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle". Nature. 323 (6090): 718–20. doi:10.1038/323718a0. PMID 2430185.
- ↑ Pedarzani P, D'hoedt D, Doorty KB, Wadsworth JD, Joseph JS, Jeyaseelan K, Kini RM, Gadre SV, Sapatnekar SM, Stocker M, Strong PN (2002). "Tamapin, a venom peptide from the Indian red scorpion (Mesobuthus tamulus) that targets small conductance Ca2+-activated K+ channels and afterhyperpolarization currents in central neurons". J. Biol. Chem. 277 (48): 46101–9. doi:10.1074/jbc.M206465200. PMID 12239213.
- 1 2 Dilly S, Lamy C, Marrion NV, Liégeois JF, Seutin V (August 2011). "Ion-channel modulators: more diversity than previously thought". Chembiochem. 12 (12): 1808–12. doi:10.1002/cbic.201100236. PMID 21726033.
- ↑ Pedarzani P, Mosbacher J, Rivard A, Cingolani LA, Oliver D, Stocker M, Adelman JP, Fakler B (2001). "Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels". J. Biol. Chem. 276 (13): 9762–9. doi:10.1074/jbc.M010001200. PMID 11134030.
- ↑ Strøbaek D, Teuber L, Jørgensen TD, Ahring PK, Kjaer K, Hansen RS, Olesen SP, Christophersen P, Skaaning-Jensen B (2004). "Activation of human IK and SK Ca2+-activated K+ channels by NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime)". Biochim. Biophys. Acta. 1665 (1–2): 1–5. doi:10.1016/j.bbamem.2004.07.006. PMID 15471565.
- ↑ Hougaard C, Eriksen BL, Jørgensen S, Johansen TH, Dyhring T, Madsen LS, Strøbaek D, Christophersen P (2007). "Selective positive modulation of the SK3 and SK2 subtypes of small conductance Ca2+-activated K+ channels". Br. J. Pharmacol. 151 (5): 655–65. doi:10.1038/sj.bjp.0707281. PMC 2014002
 . PMID 17486140.
. PMID 17486140. - ↑ Tzounopoulos T, Stackman R (December 2003). "Enhancing synaptic plasticity and memory: a role for small-conductance Ca2+-activated K+ channels". Neuroscientist. 9 (6): 434–9. doi:10.1177/1073858403259282. PMID 14678575.
- 1 2 3 Liu XK, Wang G, Chen SD (June 2010). "Modulation of the activity of dopaminergic neurons by SK channels: a potential target for the treatment of Parkinson's disease?". Neurosci Bull. 26 (3): 265–71. doi:10.1007/s12264-010-1217-4. PMID 20502506.
External links
- SK Potassium Channels at the US National Library of Medicine Medical Subject Headings (MeSH)
- "Calcium-Activated Potassium Channels". IUPHAR Database of Receptors and Ion Channels. International Union of Basic and Clinical Pharmacology.
- John Adelman. "Research interests: Small-conductance calcium-activated potassium channels (SK channels)". Oregon Health & Science University. Archived from the original on 2007-09-30. Retrieved 2008-01-22.