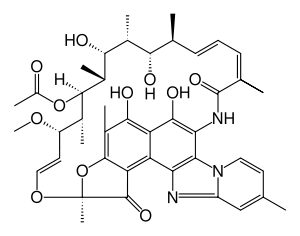
Rifaximin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Xifaxan, Xifaxanta, Normix, Rifagut |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604027 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | A07AA11 (WHO) D06AX11 (WHO) QG51AA06 (WHO) QJ51XX01 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | < 0.4% |
| Metabolism | Hepatic |
| Biological half-life | 6 hours |
| Excretion | Fecal (97%) |
| Identifiers | |
| |
| CAS Number |
80621-81-4 |
| PubChem (CID) | 6436173 |
| DrugBank |
DB01220 |
| ChemSpider |
10482302 |
| UNII |
L36O5T016N |
| KEGG |
D02554 |
| ChEBI |
CHEBI:75246 |
| ChEMBL |
CHEMBL1617 |
| ECHA InfoCard | 100.111.624 |
| Chemical and physical data | |
| Formula | C43H51N3O11 |
| Molar mass | 785.879 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Rifaximin (trade names:RCIFAX, Rifagut, Xifaxan, Zaxine) is a semisynthetic antibiotic based on rifamycin. It has poor oral bioavailability, meaning that very little of the drug will be absorbed into the blood stream when it is taken orally. Rifaximin is used in the treatment of traveler's diarrhea, irritable bowel syndrome, and hepatic encephalopathy, for which it received orphan drug status from the U.S. Food and Drug Administration in 1998.
Uses
Rifaximin is licensed by the U.S. Food and Drug Administration to treat traveler's diarrhea caused by E. coli.[1] Clinical trials have shown that rifaximin is highly effective at preventing and treating traveler's diarrhea among travelers to Mexico, with few side effects and low risk of developing antibiotic resistance.[2][3][4] It is not effective against Campylobacter jejuni, and there is no evidence of efficacy against Shigella or Salmonella species.
It may be efficacious in relieving chronic functional symptoms of bloating and flatulence that are common in irritable bowel syndrome (IBS),[5][6] especially IBS-D.
Rifaximin may also be a useful addition to vancomycin when treating patients with relapsing C. difficile infections.[7][8] Although exposure to rifamycins in the past may increase risk for resistance, so rifaximin should be avoided in such cases.
There was recently a pilot-study done on the efficacy of rifaximin as a means of treatment for rosacea, according to the study, induced by the co-presence of small intestinal bacterial overgrowth.[9]
In the United States, rifaximin has orphan drug status for the treatment of hepatic encephalopathy.[10] Although high-quality evidence is still lacking, rifaximin appears to be as effective as or more effective than other available treatments for hepatic encephalopathy (such as lactulose), is better tolerated, and may work faster.[11] Hepatic encephalopathy is a debilitating condition for those with liver disease. Rifaximin is an oral medication taken three times daily that helps patients to avoid reoccurring hepatic encephalopathy. It has minimal side effects, prevents reoccurring encephalopathy and high patient satisfaction. Patients are more compliant and satisfied to take this medication than any other due to minimal side effects, prolong remission, and overall cost.[12] Rifaximin helps patients avoid multiple readmissions from hospitals along with less time missed from work as well. Rifaximin should be considered a standard prescribed medication for those whom have episodes of hepatic encephalopathy.
The drawbacks to rifaximin are increased cost and lack of robust clinical trials for HE without combination lactulose therapy.
Also treats hyperammonemia by eradicating ammoniagenic bacteria.
Mechanism of action
Rifaximin interferes with transcription by binding to the β-subunit of bacterial RNA polymerase.[13] This results in the blockage of the translocation step that normally follows the formation of the first phosphodiester bond, which occurs in the transcription process.[14]
Efficacy
A 2011 study in patients with IBS (without constipation) indicated 11% showed benefits over a placebo.[15] The study was supported by Salix Pharmaceuticals, the patent holder.[15] A 2010 study in patients treated for hepatic cirrhosis with hospitalization involving hepatic encephalopathy resulted in 22% of the rifaxmin treated group experiencing a breakthrough episode of hepatic encephalopathy as compared to 46% of the placebo group. The majority patients were also receiving Lactulose therapy for prevention of hepatic encephalopathy in addition to Rifaximin.[16] Rifaximin shows promising results, causing remission in up to 59% of people with Crohn’s disease and up to 76% of people with ulcerative colitis.[17]
Availability
In the United States, Salix Pharmaceuticals holds a US Patent for rifaximin and markets the drug under the name Xifaxan, available in tablets of 200 mg and 550 mg.[18][19] In addition to receiving FDA approval for traveler’s diarrhea and (marketing approved for)[19] hepatic encephalopathy, Xifaxan received FDA approval for IBS in May 2015.[20] No generic formulation is available in the US and none has appeared due to the fact that the FDA approval process was ongoing. If Xifaxan receives full FDA approval for hepatic encephalopathy it is likely that Salix will maintain marketing exclusivity and be protected from generic formulations until March 24, 2017.[19] Price quotes received on February 21, 2013 for Xifaxan 550 mg in the Denver Metro area were between $23.57 and $26.72 per tablet. A price quote received on June 24, 2016 for Xifaxan 550 mg was $31.37 per tablet.
Rifaximin is approved in 33 countries for GI disorders.[21][22] On August 13, 2013, Health Canada issued a Notice of Compliance to Salix Pharmaceuticals Inc. for the drug product Zaxine.[23] In India it is available under the brand names Ciboz and Xifapill.
References
- ↑ "Xifaxan label information" (PDF). Retrieved November 15, 2008.
- ↑ DuPont, H (2007). "Therapy for and Prevention of Traveler's Diarrhea". Clinical Infectious Diseases. 45 (45 (Suppl 1)): S78–S84. doi:10.1086/518155. PMID 17582576.
- ↑ Ruiz J, Mensa L, Pons MJ, Vila J, Gascon J (May 2008). "Development of Escherichia coli rifaximin-resistant mutants: frequency of selection and stability". Journal of antimicrobial chemotherapy. 61 (5): 1016–9. doi:10.1093/jac/dkn078. PMID 18325895.
- ↑ Martinez-Sandoval F, Ericsson CD, Jiang ZD, Okhuysen PC, Romero JH, Hernandez N, Forbes WP, Shaw A, Bortey E, DuPont HL (Mar–Apr 2010). "Prevention of travelers' diarrhea with rifaximin in US travelers to Mexico.". J Travel Med. 17 (2): 111–7. doi:10.1111/j.1708-8305.2009.00385.x. PMID 20412178.
- ↑ Sharara A, Aoun E, Abdul-Baki H, Mounzer R, Sidani S, ElHajj I (2006). "A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence". Am J Gastroenterol. 101 (2): 326–33. doi:10.1111/j.1572-0241.2006.00458.x. PMID 16454838.
- ↑ Antibiotic May Help Ease Irritable Bowel, Businessweek, January 05, 2011
- ↑ Johnson S, Schriever C, Galang M, et al. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis 2007; 44:846.
- ↑ Garey KW, Ghantoji SS, Shah DN, et al. A randomized, double-blind, placebo-controlled pilot study to assess the ability of rifaximin to prevent recurrent diarrhoea in patients with Clostridium difficile infection. J Antimicrob Chemother 2011; 66:2850.
- ↑ Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Parodi A, Paolino S, Greco A, Drago F, Mansi C, Rebora A, Parodi A, Savarino V.
- ↑ Wolf, David C. (2007-01-09). "Hepatic Encephalopathy". eMedicine. WebMD. Retrieved 2007-02-15.
- ↑ Lawrence KR, Klee JA (2008). "Rifaximin for the treatment of hepatic encephalopathy". Pharmacotherapy. 28 (8): 1019–32. doi:10.1592/phco.28.8.1019. PMID 18657018. Free full text with registration at Medscape.
- ↑ Kimer, Nina; Krag, Aleksander; Gluud, Lise L. (March 2014). "Safety, efficacy, and patient acceptability of Rifaximin for hepatic encephalopathy". Patient Preference and Adherence. 8: 331–338. doi:10.2147/PPA.S41565. PMC 3964161
 . PMID 24672227.
. PMID 24672227. - ↑ http://formularyjournal.modernmedicine.com/formulary-journal/news/clinical/clinical-pharmacology/rifaximin-nonabsorbable-broad-spectrum-antibio?page=full
- ↑ http://www.drugbank.ca/drugs/DB01220
- 1 2 Pimentel, Mark; Lembo, Anthony; Chey, William D.; Zakko, Salam; Ringel, Yehuda; Yu, Jing; Mareya, Shadreck M.; Shaw, Audrey L.; Bortey, Enoch (January 2011). "Rifaximin Therapy for Patients with Irritable Bowel Syndrome without Constipation". N Engl J Med. 364 (1): 22–32. doi:10.1056/NEJMoa1004409. PMID 21208106.
- ↑ Bass NM, Mullen KD, Sanyal A et al. (March 2010). "Rifaximin treatment in hepatic encephalopathy". N Engl J Med. 362 (12): 1071–1081. doi:10.1056/NEJMoa0907893. PMID 20335583.
- ↑ Clark, Brian. "Rifaximin (Xifaxan) is a Promising Drug for the Treatment of Inflammatory Bowel Disease". Human Data Projct. Human Data Project. Retrieved 28 March 2016.
- ↑ http://www.salix.com/products/xifaxan550.aspx
- 1 2 3 http://www.accessdata.fda.gov/scripts/cder/ob/docs/obdetail.cfm?Appl_No=022554&TABLE1=OB_Rx
- ↑ http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm448328.htm
- ↑ http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/GastrointestinalDrugsAdvisoryCommittee/UCM203248.pdf
- ↑ http://www.salix.com/news-media/news/previous-years-news/fda-approves-xifaxan%C2%AE-550-mg-tablets-for-reduction-in-risk-of-overt-hepatic-encephalopathy-he-recurrence.aspx
- ↑ http://www.hc-sc.gc.ca/dhp-mps/prodpharma/sbd-smd/drug-med/sbd_smd_2013_zaxine_161256-eng.php
External links
- FDA label approved for Xifaxan (PDF warning)
- Xifaxan200 (manufacturer's website)