Bisphenol A
 | |
 | |
| Names | |
|---|---|
| IUPAC name
4,4'-(propane-2,2-diyl)diphenol | |
| Other names
BPA, p,p'-isopropylidenebisphenol, 2,2-bis(4-hydroxyphenyl)propane. | |
| Identifiers | |
| 80-05-7 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChEBI | CHEBI:33216 |
| ChEMBL | ChEMBL418971 |
| ChemSpider | 6371 |
| DrugBank | DB06973 |
| ECHA InfoCard | 100.001.133 |
| EC Number | 201-245-8 |
| 7865 | |
| KEGG | C13624 |
| PubChem | 6623 |
| RTECS number | SL6300000 |
| UNII | MLT3645I99 |
| UN number | 2430 |
| |
| |
| Properties | |
| C15H16O2 | |
| Molar mass | 228.29 g·mol−1 |
| Appearance | White solid |
| Density | 1.20 g/cm³ |
| Melting point | 158 to 159 °C (316 to 318 °F; 431 to 432 K) |
| Boiling point | 220 °C (428 °F; 493 K) 4 mmHg |
| 120–300 ppm (21.5 °C) | |
| Vapor pressure | 5×10−6 Pa (25 °C)[1] |
| Hazards | |
| R-phrases | R36 R37 R38 R43 |
| S-phrases | S24 S26 S37 |
| NFPA 704 | |
| Flash point | 227 °C (441 °F; 500 K) |
| 600 °C (1,112 °F; 873 K) | |
| Related compounds | |
| Related compounds |
phenols Bisphenol S |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
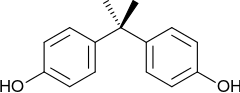
Bisphenol A (BPA) is an organic synthetic compound with the chemical formula (CH3)2C(C6H4OH)2 belonging to the group of diphenylmethane derivatives and bisphenols, with two hydroxyphenyl groups. It is a colorless solid that is soluble in organic solvents, but poorly soluble in water. It has been in commercial use since 1957.
BPA is employed to make certain plastics and epoxy resins. BPA-based plastic is clear and tough, and is made into a variety of common consumer goods, such as water bottles, sports equipment, CDs, and DVDs. Epoxy resins containing BPA are used to line water pipes, as coatings on the inside of many food and beverage cans and in making thermal paper such as that used in sales receipts.[2] In 2011, an estimated 10 billion pounds of BPA chemical were produced for manufacturing polycarbonate plastic, making it one of the highest volume of chemicals produced worldwide.[3]
BPA exhibits estrogen mimicking, hormone-like properties that raise concern about its suitability in some consumer products and food containers. Since 2008, several governments have investigated its safety, which prompted some retailers to withdraw polycarbonate products. The U.S. Food and Drug Administration (FDA) has ended its authorization of the use of BPA in baby bottles and infant formula packaging, based on market abandonment, not safety.[4] The European Union and Canada have banned BPA use in baby bottles.
The FDA states "BPA is safe at the current levels occurring in foods" based on extensive research, including two more studies issued by the agency in early 2014.[5] The European Food Safety Authority (EFSA) reviewed new scientific information on BPA in 2008, 2009, 2010, 2011 and 2015: EFSA’s experts concluded on each occasion that they could not identify any new evidence which would lead them to revise their opinion that the known level of exposure to BPA is safe; however, the EFSA does recognize some uncertainties, and will continue to investigate them.[6]
In February 2016, France announced that it intends to propose BPA as a REACH Regulation candidate substance of very high concern (SVHC).[7]
Production
World production capacity of Bisphenol A was 1 million tons in the 1980s,[8] and more than 2.2 million tons in 2009.[9] It is a high production volume chemical. In 2003, U.S. consumption was 856,000 tons, 72% of which used to make polycarbonate plastic and 21% going into epoxy resins.[10] In the U.S., less than 5% of the BPA produced is used in food contact applications,[11] but remains in the canned food industry and printing applications such as sales receipts.[12][13]
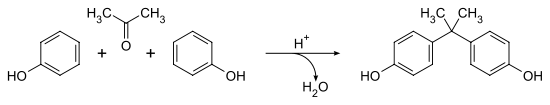
Bisphenol A was first synthesized by the Russian chemist Alexander Dianin in 1891.[14][15] This compound is synthesized by the condensation of acetone (hence the suffix A in the name)[16] with two equivalents of phenol. The reaction is catalyzed by a strong acid, such as hydrochloric acid (HCl) or a sulfonated polystyrene resin. Industrially, a large excess of phenol is used to ensure full condensation; the product mixture of the cumene process (acetone and phenol) may also be used as starting material:[8]
A large number of ketones undergo analogous condensation reactions. Commercial production of BPA requires distillation – either extraction of BPA from many resinous byproducts under high vacuum or solvent-based extraction using additional phenol followed by distillation.[8]
Uses
Bisphenol A is used primarily to make plastics, and products using bisphenol A-based plastics have been in commercial use since 1957.[17] At least 3.6 million tonnes (8 billion pounds) of BPA are used by manufacturers yearly.[18] It is a key monomer in production of epoxy resins[19][20] and in the most common form of polycarbonate plastic.[8][21][22] Bisphenol A and phosgene react to give polycarbonate under biphasic conditions; the hydrochloric acid is scavenged with aqueous base:
Diphenyl carbonate may be used in place of phosgene. Phenol is eliminated instead of hydrochloric acid. This transesterification process avoids the toxicity and handling of phosgene.[23]
Polycarbonate plastic, which is clear and nearly shatterproof, is used to make a variety of common products including baby and water bottles, sports equipment, medical and dental devices, dental fillings sealants, CDs and DVDs, household electronics, eyeglass lenses,[8] foundry castings, and the lining of water pipes.[11] BPA is also used in the synthesis of polysulfones and polyether ketones, as an antioxidant in some plasticizers, and as a polymerization inhibitor in PVC. Epoxy resins containing bisphenol A are used as coatings on the inside of almost all food and beverage cans;[24] however, due to BPA health concerns, in Japan epoxy coating was mostly replaced by PET film.[25] Bisphenol A is also a precursor to the flame retardant tetrabromobisphenol A, and was used as a fungicide.[26]
Bisphenol A is a preferred color developer in carbonless copy paper and thermal point of sale receipt paper.[27][28] When used in thermal paper, BPA is present as "free" (i.e., discrete, non-polymerized) BPA, which is likely to be more available for exposure than BPA polymerized into a resin or plastic. Upon handling, BPA in thermal paper can be transferred to skin, and there is some concern that residues on hands could be ingested through incidental hand-to-mouth contact. Furthermore, some studies suggest that dermal absorption may contribute some small fraction to the overall human exposure. European data indicate that the use of BPA in paper may also contribute to the presence of BPA in the stream of recycled paper and in landfills. Although there are no estimates for the amount of BPA used in thermal paper in the United States, in Western Europe, the volume of BPA reported to be used in thermal paper in 2005/2006 was 1,890 tonnes per year, while total production was estimated at 1,150,000 tonnes per year. (Figures taken from 2012 EPA draft paper.)[29][30] Epoxy resin may or may not contain BPA, and is employed to bind gutta percha in some root canal procedures.[31]
Identification in plastics
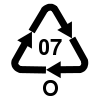
Plastic packaging is split into seven broad classes for recycling purposes by a Plastic identification code. As of 2014 there are no BPA labeling requirements for plastics in the US. "In general, plastics that are marked with Resin Identification Codes 1, 2, 3, 4, 5, and 6 are very unlikely to contain BPA. Some, but not all, plastics that are marked with the Resin Identification Code 7 may be made with BPA."[32] Type 7 is the catch-all "other" class, and some type 7 plastics, such as polycarbonate (sometimes identified with the letters "PC" near the recycling symbol) and epoxy resins, are made from bisphenol A monomer.[8][33] Type 3 (PVC) may contain bisphenol A as an antioxidant in "flexible PVC" softened by plasticizers,[8] but not rigid PVC such as pipe, windows, and siding.
History
Bisphenol A was discovered in 1891 by Russian chemist Aleksandr Dianin.[34]
Based on research by chemists at Bayer and General Electric, BPA has been used since the 1950s to harden polycarbonate plastics, and make epoxy resin, which is contained in the lining of food and beverage containers.[35][36]
In the early 1930s, the British biochemist Edward Charles Dodds tested BPA as an artificial estrogen, but found it to be 37,000 times less effective than estradiol.[37][38][39] Dodds eventually developed a structurally similar[40] compound, diethylstilbestrol (DES), which was used as a synthetic estrogen drug in women and animals until it was banned due to its risk of causing cancer; the ban on use of DES in humans came in 1971 and in animals, in 1979.[37] BPA was never used as a drug.[37] BPA's ability to mimic the effects of natural estrogen derive from the similarity of phenol groups on both BPA and estradiol, which enable this synthetic molecule to trigger estrogenic pathways in the body.[41] Typically phenol-containing molecules similar to BPA are known to exert weak oestrogenic activities, thus it is also considered an endocrine disrupter (ED) and oestrogenic chemical.[42] Xenoestrogens is another category the chemical BPA fits under because of its capability to interrupt the network that regulates the signals which control the reproductive development in humans and animals.[43]
BPA has been found to bind to both of the nuclear estrogen receptors (ERs), ERα and ERβ.[40] It is 1000- to 2000-fold less potent than estradiol.[40] The drug can both mimic the action of estrogen and antagonize estrogen, indicating that it is a selective estrogen receptor modulator (SERM) or partial agonist of the ER.[40] At high concentrations, BPA also binds to and acts as an antagonist of the androgen receptor (AR).[40] In addition to receptor binding, the compound has been found to affect Leydig cell steroidogenesis, including affecting 17α-hydroxylase/17,20 lyase and aromatase expression and interfering with LH receptor-ligand binding.[40]
In 1997, adverse effects of low-dose BPA exposure in laboratory animals were first proposed.[24] Modern studies began finding possible connections to health issues caused by exposure to BPA during pregnancy and during development. See US public health regulatory history and Chemical manufacturers reactions to bans. As of 2014, research and debates are ongoing as to whether BPA should be banned or not.
A 2007 study investigated the interaction between bisphenol A's and estrogen-related receptor γ (ERR-γ). This orphan receptor (endogenous ligand unknown) behaves as a constitutive activator of transcription. BPA seems to bind strongly to ERR-γ (dissociation constant = 5.5 nM), but only weakly to the ER.[45] BPA binding to ERR-γ preserves its basal constitutive activity.[45] It can also protect it from deactivation from the SERM 4-hydroxytamoxifen (afimoxifene).[45] This may be the mechanism by which BPA acts as a xenoestrogen.[45] Different expression of ERR-γ in different parts of the body may account for variations in bisphenol A effects. For instance, ERR-γ has been found in high concentration in the placenta, explaining reports of high bisphenol accumulation in this tissue.[44] BPA has also been found to act as an agonist of the GPER (GPR30).[46]
Safety
Health effects

According to the European Food Safety Authority "BPA poses no health risk to consumers of any age group (including unborn children, infants and adolescents) at current exposure levels".[6]
In 2012, the United States' Food and Drug Administration (FDA) banned the use of BPA in baby bottles;[47] however, the Environmental Working Group called the ban "purely cosmetic" and stated "If the agency truly wants to prevent people from being exposed to this toxic chemical associated with a variety of serious and chronic conditions it should ban its use in cans of infant formula, food and beverages." The Natural Resources Defense Council called the move inadequate, saying the FDA needed to ban BPA from all food packaging.[48] The FDA maintains that the agency continues to support the safety of BPA for use in products that hold food.[47]
The Environmental Protection Agency (EPA) also holds the position that BPA is not a health concern. In 2011, Andrew Wadge, the chief scientist of the United Kingdom's Food Standards Agency, commented on a 2011 US study on dietary exposure of adult humans to BPA,[49] saying, "This corroborates other independent studies and adds to the evidence that BPA is rapidly absorbed, detoxified, and eliminated from humans – therefore is not a health concern."[50]
The Endocrine Society said in 2015 that the results of ongoing laboratory research gave grounds for concern about the potential hazards of endocrine-disrupting chemicals – including BPA – in the environment, and that on the basis of the precautionary principle these substances should continue to be assessed and tightly regulated.[51] A 2016 review of the literature said that the potential harms caused by BPA were a topic of scientific debate and that further investigation was a priority because of the association between BPA exposure and adverse humans health effects including reproductive and developmental effects and metabolic disease.[52]
United States expert panel conclusions
In 2006, the US Government sponsored an assessment of the scientific literature on BPA. Thirty-eight experts in fields involved with bisphenol A gathered in Chapel Hill, North Carolina to review several hundred studies on BPA, many conducted by members of the group. At the end of the meeting, the group issued the Chapel Hill Consensus Statement,[53] which stated "BPA at concentrations found in the human body is associated with organizational changes in the prostate, breast, testis, mammary glands, body size, brain structure and chemistry, and behavior of laboratory animals."[54] The Chapel Hill Consensus Statement stated that average BPA levels in people were above those that cause harm to many animals in laboratory experiments. It noted that while BPA is not persistent in the environment or in humans, biomonitoring surveys indicate that exposure is continuous. This is problematic because acute animal exposure studies are used to estimate daily human exposure to BPA, and no studies that had examined BPA pharmacokinetics in animal models had followed continuous low-level exposures. The authors added that measurement of BPA levels in serum and other body fluids suggests the possibilities that BPA intake is much higher than accounted for or that BPA can bioaccumulate in some conditions (such as pregnancy).[53]
Metabolic disease
Numerous animal studies have demonstrated an association between endocrine disrupting chemicals (including BPA) and obesity.[3][55] However, the relationship between bisphenol A exposure and obesity in humans is unclear.[56] Proposed mechanisms for BPA exposure to increase the risk of obesity include BPA-induced thyroid dysfunction, activation of the PPAR-gamma receptor, and disruption of neural circuits that regulate feeding behavior.[56][57] BPA works by imitating the natural hormone 17B-estradiol. In the past BPA has been considered a weak mimicker of estrogen but newer evidence indicates that it is a potent mimicker.[58] When it binds to estrogen receptors it triggers alternative estrogenic effects that begin outside of the nucleus. This different path induced by BPA has been shown to alter glucose and lipid metabolism in animal studies.[59]
There are different effects of BPA exposure during different stages of development. During adulthood, BPA exposure modifies insulin sensitivity and insulin release without affecting weight.[60]
Thyroid function
A 2007 review concluded that bisphenol-A has been shown to bind to thyroid hormone receptor and perhaps has selective effects on its functions.[61]
A 2009 review about environmental chemicals and thyroid function raised concerns about BPA effects on triiodothyronine and concluded that "available evidence suggests that governing agencies need to regulate the use of thyroid-disrupting chemicals, particularly as such uses relate exposures of pregnant women, neonates and small children to the agents".[62]
A 2009 review summarized BPA adverse effects on thyroid hormone action.[63]
Neurological effects
Limited epidemiological evidence suggests that exposure to BPA in the uterus and during childhood is associated with poor behavioral outcomes in humans. Exposure may be associated with higher levels of anxiety, depression, hyperactivity, and aggression in children.[64] A panel convened by the National Toxicology Program (NTP) of the U.S. National Institutes of Health determined that there was "some concern" about BPA's effects on fetal and infant brain development and behavior.[10][65] In January 2010, based on the NTP report, the FDA expressed the same level of concern.[66][67]
A 2007 literature review concluded that BPA, like other chemicals that mimic estrogen (xenoestrogens), should be considered as a player within the nervous system that can regulate or alter its functions through multiple pathways.[68] A 2008 review of animal research found that low-dose BPA maternal exposure can cause long-term consequences for the neurobehavioral development in mice.[69]

A 2009 review raised concerns about a BPA effect on the anteroventral periventricular nucleus.[71]
Disruption of the dopaminergic system
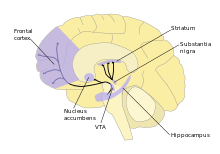
A 2008 review of human participants has concluded that BPA mimics estrogenic activity and affects various dopaminergic processes to enhance mesolimbic dopamine activity resulting in hyperactivity, attention deficits, and a heightened sensitivity to drugs of abuse.[72]
Cancer
According to the WHO's INFOSAN, carcinogenicity studies conducted under the US National Toxicology Program, have shown increases in leukemia and testicular interstitial cell tumors in male rats. However, according to the note "these studies have not been considered as convincing evidence of a potential cancer risk because of the doubtful statistical significance of the small differences in incidences from controls."[73]
A 2010 review concluded that bisphenol A may increase cancer risk.[74]
At least one study suggested that bisphenol A suppresses DNA methylation,[75] which is involved in epigenetic changes.[76]
Breast cancer

Higher susceptibility to breast cancer has been found in many studies of rodents and primates exposed to BPA.[78] However, the association between BPA and subsequent development of breast cancer in humans is unclear.[78]
Neuroblastoma

BPA promotes the growth, invasiveness and metastasis of cells from a laboratory neuroblastoma cancer cell line, SK-N-SH.[79]
Fertility
As of 2013, the evidence to support a link between BPA exposure and male infertility is weak though limited evidence does support an association with lower sperm quality.[78] There is tentative evidence to support the idea that BPA exposure has negative effects on human fertility.[78] Few studies have investigated whether recurrent miscarriage is associated with BPA levels.[78] Exposure to BPA does not appear to be linked with higher rates of endometrial hyperplasia.[78]
Sexual function
Higher BPA exposure has been associated with increased self-reporting of decreased male sexual function but few studies examining this relationship have been conducted.[78]
Asthma
Studies in mice have found a link between BPA exposure and asthma; a 2010 study on mice has concluded that perinatal exposure to 10 µg/ml of BPA in drinking water enhances allergic sensitization and bronchial inflammation and responsiveness in an animal model of asthma.[80][81] A study published in JAMA pediatrics has found that prenatal exposure to BPA is also linked to lower lung capacity in some young children. This study had 398 mother-infant pairs and looked at their urine samples to detect concentrations of BPA. They study found that every 10-fold increase in BPA was tied to a 55% increase in the odds of wheezing. The higher the concentration of BPA during pregnancy were linked to decrease lung capacity in children under four years old but the link disappeared at age 5. Associate professor of pediatrics at the University of Maryland School of Medicine said, “Exposure during pregnancy, not after, appears to be the critical time for BPA, possibly because it’s affecting important pathways that help the lung develop.”[82]
In 2013, research from scientists at the Columbia Center for Children's Environmental Health also found a link between the compound and an increased risk for asthma. The research team reported that children with higher levels of BPA at ages 3, 5 and 7 had increased odds of developing asthma when they were between the ages of 5 and 12. The children in this study had about the same concentration of BPA exposure as the average U.S child. Dr. Kathleen Donohue, an instructor at Columbia University Medical Center said, “they saw an increased risk of asthma at fairly routine, low doses of BPA.”[83] Kim Harley, who studies environmental chemicals and children's health, commented in the Scientific American journal saying while the study does not show that BPA causes asthma or wheezing, "it's an important study because we don't know a lot right now about how BPA affects immune response and asthma...They measured BPA at different ages, measured asthma and wheeze at multiple points, and still found consistent associations."[80]
Animal research
The first evidence of the estrogenicity of bisphenol A came from experiments on rats conducted in the 1930s,[39][84] but it was not until 1997 that adverse effects of low-dose exposure on laboratory animals were first reported.[24] Bisphenol A is an endocrine disruptor that can mimic estrogen and has been shown to cause negative health effects in animal studies. Bisphenol A closely mimics the structure and function of the hormone estradiol by binding to and activating the same estrogen receptor as the natural hormone.[85][86][87][88][89] Early developmental stages appear to be the period of greatest sensitivity to its effects,[90] and some studies have linked prenatal exposure to later physical and neurological difficulties.
| Dose (µg/kg/day) | Effects (measured in studies of mice or rats, descriptions (in quotes) are from Environmental Working Group)[91][92] |
Study Year |
|---|---|---|
| 0.025 | "Permanent changes to genital tract" | 2005[93] |
| 0.025 | "Changes in breast tissue that predispose cells to hormones and carcinogens" | 2005[94] |
| 1 | long-term adverse reproductive and carcinogenic effects | 2009[95] |
| 2 | "increased prostate weight 30%" | 1997[96] |
| 2 | "lower bodyweight, increase of anogenital distance in both genders, signs of early puberty and longer estrus." | 2002[97] |
| 2.4 | "Decline in testicular testosterone" | 2004[98] |
| 2.5 | "Breast cells predisposed to cancer" | 2007[99] |
| 10 | "Prostate cells more sensitive to hormones and cancer" | 2006[100] |
| 10 | "Decreased maternal behaviors" | 2002[101] |
| 30 | "Reversed the normal sex differences in brain structure and behavior" | 2003[102] |
| 50 | Adverse neurological effects occur in non-human primates | 2008[103] |
| 50 | Disrupts ovarian development | 2009[104] |
A study from 2008 concluded that blood levels of bisphenol A in neonatal mice are the same whether it is injected or ingested.[105] The current U.S. human exposure limit set by the EPA is 50 µg/kg/day.[106] In a 2010 commentary a group of scientists criticized a study designed to test low dose BPA exposure published in "Toxicological Sciences"[107] and a later editorial by the same journal,[108] which claimed the rats used in the study were insensitive to estrogen and that had other problems like the use of BPA-containing polycabonate cages[109] while the authors disagreed.[110]
Different expression of ERR-γ in different parts of the body may account for variations in bisphenol A effects. For instance, ERR-γ has been found in high concentration in the placenta, explaining reports of high bisphenol accumulation in this tissue.[44]
Environmental risk
In 2010, the U.S. Environmental Protection Agency reported that over one million pounds of BPA are released into the environment annually.[111] BPA can enter the environment either directly from chemical, plastics. coat and staining manufacturers, from paper or material recycling companies, foundries who use BPA in casting sand, or indirectly leaching from plastic, paper and metal waste in landfills.[112] or ocean-borne plastic trash.[113] Despite a soil half-life of only 1–10 days, BPA's ubiquity makes it an important pollutant; It was shown to interfere with nitrogen fixation at the roots of leguminous plants associated with the bacterial symbiont Sinorhizobium meliloti.[114]
A 2005 study conducted in the US had found that 91–98% of BPA may be removed from water during treatment at municipal water treatment plants.[115] Nevertheless, a 2009 meta-analysis of BPA in the surface water system showed BPA present in surface water and sediment in the US and Europe.[116] According to Environment Canada in 2011, "BPA can currently be found in municipal wastewater. […]initial assessment shows that at low levels, bisphenol A can harm fish and organisms over time.[117]
BPA affects growth, reproduction, and development in aquatic organisms. Among freshwater organisms, fish appear to be the most sensitive species. Evidence of endocrine-related effects in fish, aquatic invertebrates, amphibians, and reptiles has been reported at environmentally relevant exposure levels lower than those required for acute toxicity. There is a widespread variation in reported values for endocrine-related effects, but many fall in the range of 1μg/L to 1 mg/L.[11]
A 2009 review of the biological impacts of plasticizers on wildlife published by the Royal Society with a focus on aquatic and terrestrial annelids, molluscs, crustaceans, insects, fish and amphibians concluded that BPA affects reproduction in all studied animal groups, impairs development in crustaceans and amphibians and induces genetic aberrations.[118]
Positions of national and international bodies
World Health Organization
In November 2009, the WHO announced to organize an expert consultation in 2010 to assess low-dose BPA exposure health effects, focusing on the nervous and behavioral system and exposure to young children.[73] The 2010 WHO expert panel recommended no new regulations limiting or banning the use of bisphenol-A, stating that "initiation of public health measures would be premature."[119][120]
United States
In 2013, the FDA posted on its web site: "Is BPA safe? Yes. Based on FDA's ongoing safety review of scientific evidence, the available information continues to support the safety of BPA for the approved uses in food containers and packaging. People are exposed to low levels of BPA because, like many packaging components, very small amounts of BPA may migrate from the food packaging into foods or beverages."[121] FDA issued a statement on the basis of three previous reviews by a group of assembled Agency experts in 2014 in its "Final report for the review of literature and data on BPA" that said in part, "The results of these new toxicity data and studies do not affect the dose-effect level and the existing NOAEL (5 mg/kg bw/day; oral exposure)."[122]
Australia and New Zealand
In 2009 the Australia and New Zealand Food Safety Authority (Food Standards Australia New Zealand) did not see any health risk with bisphenol A baby bottles if the manufacturer's instructions were followed, as levels of exposure were very low and would not pose a significant health risk. It added that "the move by overseas manufacturers to stop using BPA in baby bottles is a voluntary action and not the result of a specific action by regulators."[123] In 2008 it had suggested the use of glass baby bottles if parents had concerns.[124]
In 2012 the Australian Government introduced a voluntary phase out of BPA use in polycarbonate baby bottles.[125]
Canada
In April 2008, Health Canada concluded that, while adverse health effects were not expected, the margin of safety was too small for formula-fed infants[126] and proposed classifying the chemical as "'toxic' to human health and the environment."[127] The Canadian Minister of Health announced Canada's intent to ban the import, sale, and advertisement of polycarbonate baby bottles containing bisphenol A due to safety concerns, and investigate ways to reduce BPA contamination of baby formula packaged in metal cans.[90] Subsequent news reports from April 2008 showed many retailers removing polycarbonate drinking products from their shelves.[128] On 18 October 2008, Health Canada noted that "bisphenol A exposure to newborns and infants is below levels that cause effects" and that the "general public need not be concerned".[129]
In 2010, Canada's department of the environment declared BPA to be a "toxic substance" and added it to schedule 1 of the Canadian Environmental Protection Act, 1999.[130]
European Union
The 2008 European Union Risk Assessment Report on bisphenol A, published by the European Commission and European Food Safety Authority (EFSA), concluded that bisphenol A-based products, such as polycarbonate plastic and epoxy resins, are safe for consumers and the environment when used as intended.[131] By October 2008, after the Lang Study was published, the EFSA issued a statement concluding that the study provided no grounds to revise the current Tolerable Daily Intake (TDI) level for BPA of 0.05 mg/kg bodyweight.[132]
On 22 December 2009, the EU Environment ministers released a statement expressing concerns over recent studies showing adverse effects of exposure to endocrine disruptors.[133]
In September 2010, the European Food Safety Authority (EFSA) concluded after a "comprehensive evaluation of recent toxicity data […] that no new study could be identified, which would call for a revision of the current TDI".[134] The Panel noted that some studies conducted on developing animals have suggested BPA-related effects of possible toxicological relevance, in particular biochemical changes in brain, immune-modulatory effects and enhanced susceptibility to breast tumours but considered that those studies had several shortcomings so the relevance of these findings for human health could not be assessed.[134]
On 25 November 2010, the European Union executive commission said it planned to ban the manufacturing by 1 March 2011 and ban the marketing and market placement of polycarbonate baby bottles containing the organic compound bisphenol A (BPA) by 1 June 2011, according to John Dalli, commissioner in charge of health and consumer policy. This was backed by a majority of EU governments.[135][136] The ban was called an over-reaction by Richard Sharpe, of the Medical Research Council's Human Reproductive Sciences Unit, who said to be unaware of any convincing evidence justifying the measure and criticized it as being done on political, rather than scientific grounds.[137]
In January 2011 use of bisphenol A in baby bottles was forbidden in all EU-countries.[138]
After reviewing more recent research, in 2012 EFSA made a decision to re-evaluate the human risks associated with exposure to BPA. They completed a draft assessment of consumer exposure to BPA in July 2013 and at that time asked for public input from all stakeholders to assist in forming a final report, which is expected to be completed in 2014.[139]
In January 2014, EFSA presented a second part of the draft opinion which discussed the human health risks posed by BPA. The draft opinion was accompanied by an eight-week public consultation and also included adverse effects on the liver and kidney as related to BPA. From this it was recommended that the current TDI to be revised.[140] In January 2015 EFSA indicated that the TDI was reduced from 50 to 4 µg/kg body weight/day – a recommendation, as national legislatures make the laws.[138]
In February 2016, France announced that it intends to propose BPA as an EU Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH Regulation) candidate substance of very high concern (SVHC) by 8 August 2016.[7] If the European Chemical Agency assigns SVHC status, the presence of BPA in a product at a concentration above 0.1% must be disclosed to a purchaser (with different rules for consumer and business purchasers). Candidate SVHC listing is a first step towards restricting the importing and use of a chemical in the EU.
Denmark
In May 2009, the Danish parliament passed a resolution to ban the use of BPA in baby bottles, which had not been enacted by April 2010. In March 2010, a temporary ban was declared by the Health Minister.[141]
Belgium
On March 2010, senator Philippe Mahoux proposed legislation to ban BPA in food contact plastics.[142] On May 2011, senators Dominique Tilmans and Jacques Brotchi proposed legislation to ban BPA from thermal paper.[143]
France
On 5 February 2010, the French Food Safety Agency (AFSSA) questioned the previous assessments of the health risks of BPA, especially in regard to behavioral effects observed in rat pups following exposure in utero and during the first months of life.[144][145] In April 2010, the AFFSA suggested the adoption of better labels for food products containing BPA.[146]
On 24 March 2010, the French Senate unanimously approved a proposition of law to ban BPA from baby bottles.[147] The National Assembly (Lower House) approved the text on 23 June 2010, which has been applicable law since 2 July 2010.[148] On 12 October 2011, the French National Assembly voted a law forbidding the use of Bisphenol A in products aimed at less than 3-year-old children for 2013, and 2014 for all food containers.[149]
On 9 October 2012, the French Senate adopted unanimously the law proposition to suspend manufacture, import, export and marketing of all food containers that include bisphenol A for 2015. The ban of bisphenol A in 2013 for food products designed for children less than 3-years-old was maintained.[150]
Germany
On 19 September 2008, the German Federal Institute for Risk Assessment (Bundesinstitut für Risikobewertung, BfR) stated that there was no reason to change the current risk assessment for bisphenol A on the basis of the Lang Study.[151]
In October 2009, the German environmental organization Bund für Umwelt und Naturschutz Deutschland requested a ban on BPA for children's products, especially pacifiers,[152] and products that make contact with food.[153] In response, some manufacturers voluntarily removed the problematic pacifiers from the market.[154]
Netherlands
On 6 November 2008, the Dutch Food and Consumer Product Safety Authority (VWA) stated in a newsletter that baby bottles made from polycarbonate plastic do not release measurable concentrations of bisphenol A and therefore are safe to use.[155]
Switzerland
In February 2009, the Swiss Federal Office for Public Health, based on reports of other health agencies, stated that the intake of bisphenol A from food represents no risk to the consumer, including newborns and infants. However, in the same statement, it advised for proper use of polycarbonate baby bottles and listed alternatives.[156]
Sweden
By May 26, 1995, the Swedish Chemicals Agency asked for a BPA ban in baby bottles, but the Swedish Food Safety Authority prefers to await the expected European Food Safety Authority's updated review. The Minister of Environment said to wait for the EFSA review but not for too long.[157][158] From March 2011 it is prohibited to manufacture babybottles containing bisphenol A and from July 2011 they can not be bought in stores. On 12 April 2012, the Swedish government announced that Sweden will ban BPA in cans containing food for children under the age of three.[159]
United Kingdom
In December 2009, responding to a letter from a group of seven scientists that urged the UK Government to "adopt a standpoint consistent with the approach taken by other Governments who have ended the use of BPA in food contact products marketed at children",[160] the UK Food Standards Agency reaffirmed, in January 2009, its view that "exposure of UK consumers to BPA from all sources, including food contact materials, was well below levels considered harmful".[161]
Turkey
As of 10 June 2011, Turkey banned the use of BPA in baby bottles and other PC items produced for babies.[162]
Japan
Between 1998 and 2003, the canning industry voluntarily replaced its BPA-containing epoxy resin can liners with BPA-free polyethylene terephthalate (PET) in many of its products. For other products, it switched to a different epoxy lining that yielded much less migration of BPA into food than the previously used resin. In addition, polycarbonate tableware for school lunches was replaced by BPA-free plastics. As a result of these changes, Japanese risk assessors have found that virtually no BPA is detectable in canned foods or drinks, and blood levels of BPA in the Japanese people have declined up to 50% in one study.[163]
Human exposure sources
The major human exposure route to BPA is diet, including ingestion of contaminated food and water.[164] Bisphenol A is leached from the lining of food and beverage cans where it is used as an ingredient in the plastic used to protect the food from direct contact with the can.[165] It is especially likely to leach from plastics when they are cleaned with harsh detergents or when they contain acidic or high-temperature liquids. BPA is used to form epoxy resin coating of water pipes; in older buildings, such resin coatings are used to avoid replacement of deteriorating pipes.[166] In the workplace, while handling and manufacturing products which contain BPA, inhalation and dermal exposures are the most probable routes.[167] There are many uses of BPA for which related potential exposures have not been fully assessed including digital media, electrical and electronic equipment, automobiles, sports safety equipment, electrical laminates for printed circuit boards, composites, paints, and adhesives.[168] In addition to being present in many products that people use on a daily basis, BPA has the ability to bioaccumulate, especially in water bodies. In one review, it was seen that although BPA is biodegradable, it is still detected after wastewater treatment in many waterways at concentrations of approximately 1 ug/L. This study also looked at other pathways where BPA could potentially bioaccumulate and found “low-moderate potential...in microorganisms, algae, invertebrates, and fish in the environment” suggesting that some environmental exposures less likely.[167]
In November 2009, the Consumer Reports magazine published an analysis of BPA content in some canned foods and beverages, where in specific cases the content of a single can of food could exceed the FDA "Cumulative Exposure Daily Intake" limit.[12][169]
The CDC had found bisphenol A in the urine of 95% of adults sampled in 1988–1994[170] and in 93% of children and adults tested in 2003–04.[171] The USEPA Reference Dose (RfD) for BPA is 50 µg/kg/day which is not enforceable but is the recommended safe level of exposure. The most sensitive animal studies show effects at much lower doses,[91][106] and several studies of children, who tend to have the highest levels, have found levels over the EPA's suggested safe limit figure.[172]
A 2009 Health Canada study found that the majority of canned soft drinks it tested had low, but measurable levels of bisphenol A.[173] A study conducted by the University of Texas School of Public Health in 2010 found BPA in 63 of 105 samples of fresh and canned foods, including fresh turkey sold in plastic packaging and canned infant formula.[174] A 2011 study published in Environmental Health Perspectives, "Food Packaging and Bisphenol A and Bis(2-Ethyhexyl) Phthalate Exposure: Findings from a Dietary Intervention," selected 20 participants based on their self-reported use of canned and packaged foods to study BPA. Participants ate their usual diets, followed by three days of consuming foods that were not canned or packaged. The study's findings include: 1) evidence of BPA in participants' urine decreased by 50% to 70% during the period of eating fresh foods; and 2) participants' reports of their food practices suggested that consumption of canned foods and beverages and restaurant meals were the most likely sources of exposure to BPA in their usual diets. The researchers note that, even beyond these 20 participants, BPA exposure is widespread, with detectable levels in urine samples in more than an estimated 90% of the U.S. population.[175] Another U.S. study found that consumption of soda, school lunches, and meals prepared outside the home were statistically significantly associated with higher urinary BPA.[172]
A 2011 experiment by researchers at the Harvard School of Public Health indicated that BPA used in the lining of food cans is absorbed by the food and then ingested by consumers. Of 75 participants, half ate a lunch of canned vegetable soup for five days, followed by five days of fresh soup, while the other half did the same experiment in reverse order. "The analysis revealed that when participants ate the canned soup, they experienced more than a 1,000 percent increase in their urinary concentrations of BPA, compared to when they dined on fresh soup."[176] A 2009 study found that drinking from polycarbonate bottles increased urinary bisphenol A levels by two thirds, from 1.2 μg/g creatinine to 2 μg/g creatinine.[177] Consumer groups recommend that people wishing to lower their exposure to bisphenol A avoid canned food and polycarbonate plastic containers (which shares resin identification code 7 with many other plastics) unless the packaging indicates the plastic is bisphenol A-free.[178] To avoid the possibility of BPA leaching into food or drink, the National Toxicology Panel recommends avoiding microwaving food in plastic containers, putting plastics in the dishwasher, or using harsh detergents.[179]
Besides diet, exposure can also occur through air and through skin absorption.[180] Free BPA is found in high concentration in thermal paper and carbonless copy paper, which would be expected to be more available for exposure than BPA bound into resin or plastic.[181][182][183][184] Popular uses of thermal paper include receipts, event and cinema tickets, labels, and airline tickets.[184] A Swiss study found that 11 of 13 thermal printing papers contained 8 – 17 g/kg bisphenol A (BPA). Upon dry finger contact with a thermal paper receipt, roughly 1 μg BPA (0.2 – 6 μg) was transferred to the forefinger and the middle finger. For wet or greasy fingers approximately 10 times more was transferred. Extraction of BPA from the fingers was possible up to 2 hours after exposure.[185] Further, it has been demonstrated that thermal receipts placed in contact with paper currency in a wallet for 24 hours cause a dramatic increase in the concentration of BPA in paper currency, making paper money a secondary source of exposure.[186] Another study has identified BPA in all of the waste paper samples analysed (newspapers, magazines, office paper, etc.), indicating direct results of contamination through paper recycling.[2] Free BPA can readily be transferred to skin, and residues on hands can be ingested.[11] Bodily intake through dermal absorption (99% of which comes from handling receipts) has been shown for the general population to be 0.219 ng/kg bw/day (occupationally exposed persons absorb higher amounts at 16.3 ng/kg bw/day)[187] whereas aggregate intake (food/beverage/environment) for adults is estimated at 0.36–0.43 μg/kg bw/day (estimated intake for occupationally exposed adults is 0.043–100 μg/kg bw/day).[10]
A study from 2011 found that Americans of all age groups had twice as much BPA in their bodies as Canadians; the reasons for the disparity were unknown, as there was no evidence to suggest higher amounts of BPA in U.S. foods, or that consumer products available in the U.S. containing BPA were BPA-free in Canada. According to another study it may have been due to differences in how and when the surveys were done,[188] because "although comparisons of measured concentrations can be made across populations, this must be done with caution owing to differences in sampling, in the analytical methods used and in the sensitivity of the assays."[189]
Comparing data from the National Health and Nutrition Examination Surveys (NHANES) from four time periods between 2003 and 2012, urinary BPA data the median daily intake for the overall population is approximately 25 ng/kg/day and below current health based guidelines. Additionally, daily intake of BPA in the United States has decreased significantly compared to the intakes measured in 2003–2004.[190] Public attention and governmental action during this time period may have decreased the exposure to BPA somewhat but these studies did not include children under the age of six. According to the Endocrine Society, age of exposure is an important factor in determining the extent to which endocrine disrupting chemicals will have an effect, and the effects on developing fetuses or infants is quite different than an adult.[191]
Fetal and early-childhood exposures
A 2009 study found higher urinary concentrations in young children than in adults under typical exposure scenarios.[192][193] In adults, BPA is eliminated from the body through a detoxification process in the liver. In infants and children, this pathway is not fully developed so they have a decreased ability to clear BPA from their systems. Several recent studies of children have found levels that exceed the EPAs suggested safe limit figure.[172]
Infants fed with liquid formula are among the most exposed, and those fed formula from polycarbonate bottles can consume up to 13 micrograms of bisphenol A per kg of body weight per day (μg/kg/day; see table below).[194] In the US and Canada, BPA has been found in infant liquid formula in concentrations varying from 0.48 to 11 ng/g.[195][196] BPA has been rarely found in infant powder formula (only 1 of 14).[195] The U.S. Department of Health & Human Services (HHS) states that "the benefit of a stable source of good nutrition from infant formula and food outweighs the potential risk of BPA exposure".[197] BPA is present in human breast milk, having been found by several studies in 62–75% of breast milk samples.[198][199]
Children may be more susceptible to BPA exposure than adults (see health effects). A 2010 study of people in Austria, Switzerland, and Germany has suggested polycarbonate (PC) baby bottles as the most prominent role of exposure for infants, and canned food for adults and teenagers.[200] In the United States, the growing concern over BPA exposure in infants in recent years has led the manufacturers of plastic baby bottles to stop using BPA in their bottles. The FDA banned the use of BPA in baby bottles and sippy cups (July 2012) as well as the use of epoxy resins in infant formula packaging.[201] However, babies may still be exposed if they are fed with old or hand-me-down bottles bought before the companies stopped using BPA.
One often overlooked source of exposure occurs when a pregnant woman is exposed, thereby exposing the fetus. Animal studies have shown that BPA can be found in both the placenta and the amniotic fluid of pregnant mice.[202] Since BPA was also "detected in the urine and serum of pregnant women and the serum, plasma, and placenta of newborn infants" a study to examine the externalizing behaviors associated with prenatal exposure to BPA was performed which suggests that exposures earlier in development have more of an effect on the behavior outcomes and that female children (2-years-old) are impacted more than males.[203] A small US study in 2009, funded by the Environmental Working Group, detected an average of 2.8 ng/mL BPA in the blood of 9 out of the 10 umbilical cords tested.[204] A study of 244 mothers indicated that exposure to BPA before birth could affect the behavior of girls at age 3. Girls whose mother's urine contained high levels of BPA during pregnancy scored worse on tests of anxiety and hyperactivity. Although these girls still scored within a normal range, for every 10-fold increase in the BPA of the mother, the girls scored at least six points lower on the tests. Boys did not seem to be affected by their mother's BPA levels during pregnancy.[205] After the baby is born, maternal exposure can continue to affect the infant through transfer of BPA to the infant via breast milk.[206][207] Because of these exposures that can occur both during and after pregnancy, mothers wishing to limit their child's exposure to BPA should attempt to limit their own exposures during that time period.
While the majority of exposures have been shown to come through the diet, accidental ingestion can also be considered a source of exposure. One study conducted in Japan tested plastic baby books to look for possible leaching into saliva when babies chew on them.[208] While the results of this study have yet to be replicated, it gives reason to question whether exposure can also occur in infants through ingestion by chewing on certain books or toys.
| Population | Estimated daily bisphenol A intake (μg/kg body weight/day). Table adapted from the National Toxicology Program Expert Panel Report.[10] |
|---|---|
| Infant (0–6 months) formula-fed (lower number assumes weight of 4.5 kg and intake of 700 ml/day with maximum concentration of BPA detected in U.S. canned formula; higher number assumes weight of 6.1 kg and intake of 1060 ml/day from powdered formula in cans with epoxy linings and using polycarbonate bottles) |
|
| Infant (0–6 months) breast-fed (lower number assumes weight of 6.1 kg and intake of 1060 ml/day with maximum concentration of BPA detected in Japanese breast milk samples; higher number assumes weight of 4.5 kg and intake of 700 ml/day with maximum concentration of free BPA detected in U.S. breast milk samples) |
|
| Infant (6–12 months) | |
| Child (1.5–6 years) | |
| Adult (general population) | |
| Adult (occupational) | |
Regulation
US public health regulatory history
Charles Schumer introduced a 'BPA-Free Kids Act of 2008' to the U.S. Senate seeking to ban BPA in any product designed for use by children and require the Center for Disease Control to conduct a study about the health effects of BPA exposure.[209] It was reintroduced in 2009 in both Senate and House, but died in committee each time.[111]
In 2008, the FDA reassured consumers that current limits were safe, but convened an outside panel of experts to review the issue. The Lang study was released, and co-author David Melzer presented the results of the study before the FDA panel.[210] An editorial accompanying the Lang study's publication criticized the FDA's assessment of bisphenol A: "A fundamental problem is that the current ADI [acceptable daily intake] for BPA is based on experiments conducted in the early 1980s using outdated methods (only very high doses were tested) and insensitive assays. More recent findings from independent scientists were rejected by the FDA, apparently because those investigators did not follow the outdated testing guidelines for environmental chemicals, whereas studies using the outdated, insensitive assays (predominantly involving studies funded by the chemical industry) are given more weight in arriving at the conclusion that BPA is not harmful at current exposure levels."[89] The FDA was criticized that it was "basing its conclusion on two studies while downplaying the results of hundreds of other studies."[210] Diana Zuckerman, president of the National Research Center for Women and Families, criticized the FDA in her testimony at the FDA's public meeting on the draft assessment of bisphenol A for use in food contact applications, that "At the very least, the FDA should require a prominent warning on products made with BPA".[211][212]
In March 2009 Suffolk County, New York became the first county to pass legislation to ban baby beverage containers made with bisphenol A.[213] By March 2009, legislation to ban bisphenol A had been proposed in both House and Senate.[214] In the same month, Rochelle Tyl, author of two studies used by FDA to assert BPA safety in August 2008, said those studies did not claim that BPA is safe, because they were not designed to cover all aspects of the chemical's effects.[215] In May 2009, Minnesota and Chicago were the first US jurisdictions to pass regulations limiting or banning BPA.[216][217] In June 2009, the FDA announced its decision to reconsider the BPA safety levels.[218] Grassroots political action led Connecticut to become the first US state to ban bisphenol A not only from infant formula and baby food containers, but also from any reusable food or beverage container.[219] In July 2009, the California Environmental Protection Agency's Developmental and Reproductive Toxicity Identification Committee in the California Office of Environmental Health Hazard Assessment unanimously voted against placing Bisphenol A on the state's list of chemicals that are believed to cause reproductive harm. The panel was concerned over the growing scientific evidence showing BPA's reproductive harm in animals, found that there was insufficient data of the effects in humans.[220] Critics pointed out that the same panel failed to add second-hand smoke to the list until 2006, and only one chemical was added to the list in the last three years.[221] In September, the US Environmental Protection Agency announced that it was evaluating BPA for an action plan development.[222] In October, the NIH announced $30,000,000 in stimulus grants to study the health effects of BPA. This money was supposed to result in many peer-reviewed publications.[223]
On 15 January 2010, the FDA expressed "some concern", the middle level in its scale of concerns, about the potential effects of BPA on the brain, behavior, and prostate gland in fetuses, infants, and young children, and announced that it was taking reasonable steps to reduce human exposure to BPA in the food supply. However, the FDA was not recommending that families change the use of infant formula or foods, as it saw the benefit of a stable source of good nutrition as outweighing the potential risk from BPA exposure.[224] On the same date, the Department of Health and Human Services released information to help parents to reduce children's BPA exposure.[225] As of 2010 many US states were considering some sort of BPA ban.[226]
In June 2010 the 2008–2009 Annual Report of the President's Cancer Panel was released and recommended: "Because of the long latency period of many cancers, the available evidence argues for a precautionary approach to these diverse chemicals, which include (…) bisphenol A".[227] In August 2010, the Maine Board of Environmental Protection voted unanimously to ban the sale of baby bottles and other reusable food and beverage containers made with bisphenol A as of January 2012.[228] In February 2011, the newly elected governor of Maine, Paul LePage, gained national attention when he spoke on a local TV news show saying he hoped to repeal the ban because, "There hasn't been any science that identifies that there is a problem" and added: "The only thing that I've heard is if you take a plastic bottle and put it in the microwave and you heat it up, it gives off a chemical similar to estrogen. So the worst case is some women may have little beards."[229][230] In April 2011, the Maine legislature passed a bill to ban the use of BPA in baby bottles, sippy cups, and other reusable food and beverage containers, effective 1 January 2012. Governor LePage refused to sign the bill.[231]
In October 2011, California banned BPA from baby bottles and toddlers' drinking cups, effective 1 July 2013.[232] By 2011, 26 states had proposed legislation that would ban certain uses of BPA. Many bills died in committee.[233] In July 2011, the American Medical Association (AMA) declared feeding products for babies and infants that contain BPA should be banned. It recommended better federal oversight of BPA and clear labeling of products containing it. It stressed the importance of the FDA to "actively incorporate current science into the regulation of food and beverage BPA-containing products."[234]
In 2012, the FDA concluded an assessment of scientific research on the effects of BPA and stated in the March 2012 Consumer Update that "the scientific evidence at this time does not suggest that the very low levels of human exposure to BPA through the diet are unsafe" although recognizing "potential uncertainties in the overall interpretation of these studies including route of exposure used in the studies and the relevance of animal models to human health. The FDA is continuing to pursue additional research to resolve these uncertainties."[235] Yet on 17 July 2012, the FDA banned BPA from baby bottles and sippy cups. A FDA spokesman said the agency's action was not based on safety concerns and that "the agency continues to support the safety of BPA for use in products that hold food."[236] Since manufacturers had already stopped using the chemical in baby bottles and sippy cups, the decision was a response to a request by the American Chemistry Council, the chemical industry's main trade association, who believed that a ban would boost consumer confidence.[237] The ban was criticized as "purely cosmetic" by the Environmental Working Group, which stated that "If the agency truly wants to prevent people from being exposed to this toxic chemical associated with a variety of serious and chronic conditions it should ban its use in cans of infant formula, food and beverages." The Natural Resources Defense Council called the move inadequate saying, the FDA needs to ban BPA from all food packaging.[48]
As of 2014, 12 states have banned BPA from children's bottles and feeding containers.[238]
Environmental regulation in the United States
On 30 December 2009 EPA released a so-called action plan for four chemicals, including BPA, which would have added it to the list of "chemicals of concern" regulated under the Toxic Substances Control Act. In February 2010, after lobbyists for the chemical industry had met with administration officials, the EPA delayed BPA regulation by not including the chemical.[239][240]
On 29 March 2010, EPA published a revised action plan for BPA as "chemical of concern".[241] In October 2010 an advanced Notice of Proposed Rulemaking for BPA testing was published in the Federal Register July 2011.[242] After more than 3 years at the Office of Information and Regulatory Affairs (OIRA), part of the Office of Management and Budget (OMB), which has to review draft proposals within 3 months, OIRA had not done so.
In September 2013 EPA withdrew its 2010 draft BPA rule.[243] saying the rule was “no longer necessary”, because EPA was taking a different track at looking at chemicals, a so-called "Work Plan" of more than 80 chemicals for risk assessment and risk reduction. Another proposed rule that EPA withdrew would have limited industry's claims of confidential business information (CBI) for the health and safety studies needed, when new chemicals are submitted under TSCA for review. The EPA said it continued "to try to reduce unwarranted claims of confidentiality and has taken a number of significant steps that have had dramatic results... tightening policies for CBI claims and declassifying unwarranted confidentiality claims, challenging companies to review existing CBI claims to ensure that they are still valid and providing easier and enhanced access to a wider array of information.”
The chemical industry group American Chemistry Council commended EPA for "choosing a course of action that will ultimately strengthen the performance of the nation’s primary chemical management law.” Richard Denison, senior scientist with the Environmental Defense Fund, commented “both rules were subject to intense opposition and lobbying from the chemical industry” and “Faced presumably with the reality that [the Office of Information and Regulatory Affairs] was never going to let EPA even propose the rules for public comment, EPA decided to withdraw them.”[244]
On 29 January 2014 EPA released a final alternatives assessment for BPA in thermal paper as part of its Design for the Environment program.[245]
Chemical manufacturers reactions to bans
In March 2009 the six largest US producers of baby bottles decided to stop using bisphenol A in their products.[246] The same month Sunoco, a producer of gasoline and chemicals, refused to sell BPA to companies for use in food and water containers for children younger than 3, saying it could not be certain of the compound's safety.[247] In May 2009, Lyndsey Layton from the Washington Post accused manufacturers of food and beverage containers and some of their biggest customers of the public relations and lobbying strategy to block government BPA bans. She noted that, "Despite more than 100 published studies by government scientists and university laboratories that have raised health concerns about the chemical, the Food and Drug Administration has deemed it safe largely because of two studies, both funded by a chemical industry trade group".[248] In August 2009 the Milwaukee Journal Sentinel investigative series into BPA and its effects showed the Society of the Plastics Industry plans of a major public relations blitz to promote BPA, including plans to attack and discredit those who report or comment negatively on BPA and its effects.[249][250]
BPA free, unknown substitute
The chemical industry over time responded to criticism of BPA by promoting "BPA-free" products. For example, in 2010, General Mills announced it had found a "BPA-free alternative" can liner that works with tomatoes. It said it would begin using the BPA-free alternative in tomato products sold by its organic foods subsidiary Muir Glen with that year's tomato harvest.[251] As of 2014, General Mills has refused to state which alternative chemical it uses, and whether it uses it on any of its other canned products.[252]
BPA free, epoxyfree
A minority of companies have stated what alternative compound(s) they use. Following an inquiry by Representative Edward Markey (D-Mass)[252] seventeen companies replied, xxx said they were going BPA-free. None of the companies said they are or were going to use Bisphenol S; only four stated the alternative to BPA that they will be using. ConAgra stated in 2013 "alternate liners for tomatoes are vinyl...New aerosol cans are lined with polyester resin", Eden Foods stated that only their "beans are canned with a liner of an "oleoresinous c-enamel that does not contain the endocrine disruptor BPA. Oleoresin is a mixture of oil and resin extracted from plants such as pine or balsam fir", Hain Celestial Group will use "modified polyester and/ or acrylic … by June 2014 for our canned soups, beans, and vegetables", Heinz stated in 2011 it "intend[s] to replace epoxy linings in all our food containers…. We have prioritized baby foods", and in 2012 "no BPA in any plastic containers we use".[252]
BPA substitute BPS
Some "BPA free" plastics are made from epoxy containing a compound called bisphenol S (BPS). BPS shares a similar structure and versatility to BPA and has been used in numerous products from currency to thermal receipt paper. Widespread human exposure to BPS was confirmed in an analysis of urine samples taken in the U.S., Japan, China, and five other Asian countries.[253] Researchers found BPS in all the receipt paper, 87 percent of the paper currency and 52 percent of recycled paper they tested. The study found that people may be absorbing 19 times more BPS through their skin than the amount of BPA they absorbed, when it was more widely used.[254] In a 2011 study researchers looked at 455 common plastic products and found that 70% tested positive for estrogenic activity. After the products had been washed or microwaved the proportion rose to 95%. The study concluded: "Almost all commercially available plastic products we sampled, independent of the type of resin, product, or retail source, leached chemicals having reliably-detectable EA [endocrine activity], including those advertised as BPA-free. In some cases, BPA-free products released chemicals having more EA than BPA-containing products."[254] A systematic review published in 2015 found that "based on the current literature, BPS and BPF are as hormonally active as BPA, and have endocrine disrupting effects."[255]
Phenol-based substitutes
Among potential substitutes for BPA, phenol-based chemicals closely related to BPA have been identified. The non-extensive list includes bisphenol E (BPE), bisphenols B (BPB), 4-cumylphenol (HPP) and bisphenol F (BPF).[2]
Bioremediation
Microbial degradation
Two enzymes participate in bisphenol degradation:
- The enzyme 2,4'-dihydroxyacetophenone dioxygenase, which can be found in Alcaligenes sp, transforms 2,4'-dihydroxyacetophenone and O2 into 4-hydroxybenzoate and formate.[256]
- The enzyme 4-hydroxyacetophenone monooxygenase, which can be found in Pseudomonas fluorescens, uses (4-hydroxyphenyl)ethan-1-one, NADPH, H+ and O2 to produce 4-hydroxyphenyl acetate, NADP+, and H2O.[257]
The fungus Cunninghamella elegans is also able to degrade synthetic phenolic compounds like bisphenol A.[258]
Plant degradation
Portulaca oleracea efficiently removes bisphenol A from a hydroponic solution. How this happens is unclear.[259]
References
- ↑ "Chemical Fact Sheet – Cas #80057 CASRN 80-05-7". speclab.com. 1 April 2012.
- 1 2 3 Pivnenko, K.; Pedersen, G. A.; Eriksson, E.; Astrup, T. F. (2015-10-01). "Bisphenol A and its structural analogues in household waste paper". Waste Management. 44: 39–47. doi:10.1016/j.wasman.2015.07.017. PMID 26194879.
- 1 2 vom Saal, Frederick S.; Nagel, Susan C.; Coe, Benjamin L.; Angle, Brittany M.; Taylor, Julia A. (2012-05-06). "The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity". Molecular and Cellular Endocrinology. Environment, Epigenetics and Reproduction. 354 (1–2): 74–84. doi:10.1016/j.mce.2012.01.001. PMC 3306519
 . PMID 22249005.
. PMID 22249005. - ↑ "FDA Regulations No Longer Authorize the Use of BPA in Infant Formula Packaging Based on Abandonment; Decision Not Based on Safety". Fda.gov. 2013-07-12. Retrieved 2014-02-01.
- ↑ "Bisphenol A (BPA): Use in Food Contact Application". fda.gov.
- 1 2 "Bisphenol A". European Food Safety Authority. 2015. Lay summary.
- 1 2 "Current SVHC Intentions – Bisphenol A".
- 1 2 3 4 5 6 7 Fiege H; Voges H-W; Hamamoto T; Umemura S; = Iwata T; Miki H; Fujita Y; Buysch H-J; = Garbe D; Paulus W (2002). Phenol Derivatives. Ullmann's Encyclopedia of Industrial Chemistry. Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_313.
- ↑ "Experts demand European action on plastics chemical". Reuters. 22 June 2010.
- 1 2 3 4 National Toxicology Program, U.S. Department of Health and Human Services (September 2008). "CERHR Expert Panel Report for Bisphenol A" (PDF). Retrieved 2013-05-31.
- 1 2 3 4 "Bisphenol A Action Plan" (PDF). U.S. Environmental Protection Agency. 29 March 2010. Retrieved 12 April 2010.
- 1 2 "Concern over canned foods". Consumer Reports. December 2009. Retrieved 2 February 2012.
- ↑ "Soaring BPA Levels Found in People Who Eat Canned Foods". Fox News Channel. 23 November 2011.
- ↑ А. П. Дианин (A. P. Dianin) (1891). "О продуктах конденсации кетонов с фенолами" [On condensation products of ketones with phenols]. Журнал Русского Физико-Химического Общества (Journal of the Russian Physical-Chemical Society). 23: 488–517, 523–546, 601–611. See especially p. 492.
- ↑ Zincke, T. (1905). "Ueber die Einwirkung von Brom und von Chlor auf Phenole: Substitutionsprodukte, Pseudobromide und Pseudochloride". Justus Liebigs Annalen der Chemie. 343: 75–99. doi:10.1002/jlac.19053430106.
- ↑ Uglea, Constantin V.; Negulescu, Ioan I. (1991). Synthesis and Characterization of Oligomers. CRC Press. p. 103. ISBN 0-8493-4954-0.
- ↑ "Bisphenol A Information Sheet" (PDF). Bisphenol A Global Industry Group. October 2002. Retrieved 7 December 2010.
- ↑ "Studies Report More Harmful Effects From BPA". U.S. News & World Report. 10 June 2009. Retrieved 28 October 2010.
- ↑ Replogle J (17 July 2009). "Lawmakers to press for BPA regulation". California Progress Report. Retrieved 31 January 2012.
- ↑ Ubelacker, Sheryl (16 April 2008). "Ridding life of bisphenol A a challenge". Toronto Star. Retrieved 2 August 2009.
- ↑ Kroschwitz, Jacqueline I. Kirk-Othmer encyclopedia of chemical technology. 5 (5 ed.). p. 8. ISBN 0-471-52695-9.
- ↑ "Polycarbonate (PC) Polymer Resin". Alliance Polymers, Inc. Retrieved 2 August 2009.
- ↑ Wittcoff, Harold; Reuben, B. G.; Plotkin, Jeffrey S. (2004). Industrial Organic Chemicals. Wiley-IEEE. p. 278. ISBN 978-0-471-44385-8. Retrieved 1 February 2012.
- 1 2 3 Erickson BE (2 June 2008). "Bisphenol A under scrutiny". Chemical and Engineering News. American Chemical Society. 86 (22): 36–39. doi:10.1021/cen-v086n022.p036.
- ↑ Byrne, Jane (22 September 2008). "Consumers fear the packaging – a BPA alternative is needed now". Retrieved 5 January 2010.
- ↑ "Bisphenol A". Pesticideinfo.org. Retrieved 23 October 2011.
- ↑ US 6562755, "Thermal paper with security features"
- ↑ Raloff, Janet (7 October 2009). "Concerned about BPA: Check your receipts". Science News. Retrieved 3 August 2010.
- ↑ "Archived copy" (PDF). Archived from the original (PDF) on 28 January 2013. Retrieved 2013-01-23.
- ↑ "Safer Choice" (PDF). epa.gov.
- ↑ Marciano MA, Ordinola-Zapata R, Cunha TV, Duarte MA, Cavenago BC, Garcia RB, Bramante CM, Bernardineli N, Moraes IG (April 2011). "Analysis of four gutta-percha techniques used to fill mesial root canals of mandibular molars". International Endodontic Journal. 44 (4): 321–329. doi:10.1111/j.1365-2591.2010.01832.x. PMID 21219361.
- ↑ "Bisphenol A (BPA) Information for Parents". Hhs.gov. 15 January 2010. Retrieved 23 October 2011.
- ↑ Biello D (19 February 2008). "Plastic (not) fantastic: Food containers leach a potentially harmful chemical". Scientific American. 2. Retrieved 9 April 2008.
- ↑ See:
- А. Дианина (1891) "О продуктахъ конденсацiи кетоновъ съ фенолами" (On condensation products of ketones with phenols), Журнал Русского физико-химического общества (Journal of the Russian Physical Chemistry Society), 23 : 488-517, 523–546, 601–611 ; see especially pages 491-493 ("Диметилдифеиолметаиь" (dimethyldiphenolmethane)).
- Reprinted in condensed form in: A. Dianin (1892) "Condensationsproducte aus Ketonen und Phenolen" (Condensation products of ketones and phenols), Berichte der Deutschen chemischen Gesellschaft zu Berlin, 25, part 3 : 334-337.
- ↑ Heather Caliendo for PlasticsToday – Packaging Digest, June 20, 2012 History of BPA Archived 12 June 2013 at the Wayback Machine.
- ↑ Walsh B (1 April 2010). "The Perils of Plastic – Environmental Toxins – TIME". Time. Retrieved 2 July 2010.
- 1 2 3 Vogel SA (2009). "The Politics of Plastics: The Making and Unmaking of Bisphenol A "Safety". Am J Public Health. 99 (S3): S559–S566. doi:10.2105/AJPH.2008.159228. PMC 2774166
 . PMID 19890158.
. PMID 19890158. - ↑ Dodds EC, Lawson W (1936). "Synthetic Œstrogenic Agents without the Phenanthrene Nucleus". Nature. 137 (3476): 996. Bibcode:1936Natur.137..996D. doi:10.1038/137996a0.
- 1 2 Dodds E. C.; Lawson W. "Molecular Structure in Relation to Oestrogenic Activity. Compounds without a Phenanthrene Nucleus". Proceedings of the Royal Society of London, Series B, Biological Sciences. 125 (839): 222–232. doi:10.1098/rspb.1938.0023.
- 1 2 3 4 5 6 Hejmej, Anna; Kotula-Balak, Magorzata; Bilinsk, Barbara (2011). "Antiandrogenic and Estrogenic Compounds: Effect on Development and Function of Male Reproductive System". Steroids – Clinical Aspect. doi:10.5772/28538.
- ↑ Kwon J.H.; Katz L.E.; Liljestrand H.M. (2007). "Modeling binding equilibrium in a competitive estrogen receptor binding assay". Chemosphere. 69 (7): 1025–1031. doi:10.1016/j.chemosphere.2007.04.047. PMID 17559906.
- ↑ Ahmed, R. A. M. (2014). "Effect of prenatal exposure to Bisphenol A on the vagina of albino rats: immunohistochemical and ultrastructural study". Folia Morphologica. 73: 399–408. doi:10.5603/FM.2014.0061.
- ↑ Ramos, J.G. (2003). "Bisphenol A induces both transient and permanent histofunctional alterations of the hypothalamic-pituitary-gonadal axis in prenatally exposed male rats.". Endocrinology. 144: 3206–3215. doi:10.1210/en.2002-0198.
- 1 2 3 Takeda Y.; Liu X.; Sumiyoshi M.; Matsushima A.; Shimohigashi M.; Shimohigashi Y. (July 2009). "Placenta expressing the greatest quantity of bisphenol A receptor ERR{gamma} among the human reproductive tissues: Predominant expression of type-1 ERRgamma isoform". J. Biochem. 146 (1): 113–22. doi:10.1093/jb/mvp049. PMID 19304792.
- 1 2 3 4 Matsushima A, Kakuta Y, Teramoto T, Koshiba T, Liu X, Okada H, Tokunaga T, Kawabata S, Kimura M, Shimohigashi Y (October 2007). "Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR gamma". J. Biochem. 142 (4): 517–24. doi:10.1093/jb/mvm158. PMID 17761695.
- ↑ Prossnitz, Eric R.; Barton, Matthias (2014). "Estrogen biology: New insights into GPER function and clinical opportunities". Molecular and Cellular Endocrinology. 389 (1-2): 71–83. doi:10.1016/j.mce.2014.02.002. ISSN 0303-7207. PMC 4040308
 . PMID 24530924.
. PMID 24530924. - 1 2 Mirmira, P; Evans-Molina, C. "Bisphenol A, obesity, and type 2 diabetes mellitus: genuine concern or unnecessary preoccupation?". Translational research: the journal of laboratory and clinical medicine (Review). 164 (1): 13–21. doi:10.1016/j.trsl.2014.03.003. PMC 4058392
 . PMID 24686036.
. PMID 24686036. - 1 2 "FDA to Ban BPA from Baby Bottles; Plan Falls Short of Needed Protections: Scientists". Common Dreams.
- ↑ Teeguarden JG, Calafat AM, Ye X, Doerge DR, Churchwell MI, Gunawan R, Graham MK (September 2011). "Twenty-four hour human urine and serum profiles of bisphenol A during high-dietary exposure". Toxicological Sciences. 123 (1): 48–57. doi:10.1093/toxsci/kfr160. PMID 21705716.
- ↑ Wage, Andrew (27 July 2011). "Small pond, same big issues". FSA. Retrieved 3 August 2011.
- ↑ Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT (2015). "Executive Summary to EDC-2: The Endocrine Society's Second Scientific Statement on Endocrine-Disrupting Chemicals". Endocr. Rev. 36 (6): 593–602. doi:10.1210/er.2015-1093. PMID 26414233.
- ↑ Giulivo M, Lopez de Alda M, Capri E, Barceló D (2016). "Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review". Environ. Res. (Review). 151: 251–264. doi:10.1016/j.envres.2016.07.011. PMID 27504873.
- 1 2 Vom Saal FS; et al. (2007). "Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure". Reprod Toxicol. 24 (2): 131–8. doi:10.1016/j.reprotox.2007.07.005. PMC 2967230
 . PMID 17768031.
. PMID 17768031. - ↑ Vogel S (2009). "The Politics of Plastics: The Making and Unmaking of Bisphenol A 'Safety'". American Journal of Public Health. 99 (S3): 559–566. doi:10.2105/AJPH.2008.159228. PMC 2774166
 . PMID 19890158.
. PMID 19890158. - ↑ Bhandari, Ruchi; Xiao, Jie; Shankar, Anoop (2013-06-01). "Urinary Bisphenol A and Obesity in US Children". American Journal of Epidemiology. 177 (11): 1263–1270. doi:10.1093/aje/kws391. ISSN 0002-9262. PMC 3664337
 . PMID 23558351.
. PMID 23558351. - 1 2 Oppeneer, SJ; Robien, K (July 2015). "Bisphenol A exposure and associations with obesity among adults: a critical review". Public Health Nutrition (Systematic Review). 18 (10): 1847–63. doi:10.1017/S1368980014002213. PMID 25311796.
- ↑ Rezg R, El-Fazaa S, Gharbi N, Mornagui B (March 2014). "Bisphenol A and human chronic diseases: Current evidences, possible mechanisms, and future perspectives". Environment International. 64: 83–90. doi:10.1016/j.envint.2013.12.007. PMID 24382480.
- ↑ Alonso-Magdalena, P; Ropero, AB; Soriano, S; García-Arévalo, M; Ripoll, C; Fuentes, E; Quesada, I; Nadal, Á (22 May 2012). "Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways.". Molecular and cellular endocrinology. 355 (2): 201–7. doi:10.1016/j.mce.2011.12.012. PMID 22227557.
- ↑ Alonso-Magdalena, P; Ropero, AB; Soriano, S; Quesada, I; Nadal, A (2010). "Bisphenol-A: a new diabetogenic factor?". Hormones (Athens, Greece). 9 (2): 118–26. PMID 20687395.
- ↑ Alonso-Magdalena P, Quesada I, Nadal A (June 2011). "Endocrine disruptors in the etiology of type 2 diabetes mellitus". Nat Rev Endocrinol. 7 (6): 346–53. doi:10.1038/nrendo.2011.56. PMID 21467970.
- 1 2 Zoeller RT (2007). "Environmental chemicals impacting the thyroid: Targets and consequences". Thyroid. 17 (9): 811–7. doi:10.1089/thy.2007.0107. PMID 17956155.
- 1 2 Boas M, Main KM, Feldt-Rasmussen U (2009). "Environmental chemicals and thyroid function: An update". Current opinion in endocrinology, diabetes, and obesity. 16 (5): 385–91. doi:10.1097/MED.0b013e3283305af7. PMID 19625957.
- ↑ Kashiwagi, Keiko; Furuno, Nobuaki; Kitamura, Shigeyuki; Ohta, Shigeru; Sugihara, Kazumi; Utsumi, Kozo; Hanada, Hideki; Taniguchi, Kikuyo; Suzuki, Ken-Ichi; Kashiwagi, Akihiko (2009). "Disruption of Thyroid Hormone Function by Environmental Pollutants". Journal of Health Science. 55 (2): 147–160. doi:10.1248/jhs.55.147.
- ↑ Ejaredar, M; Lee, Y; Roberts, DJ; Sauve, R; Dewey, D (March 2016). "Bisphenol A exposure and children's behavior: A systematic review". Journal of Exposure Science & Environmental Epidemiology (Systematic Review). doi:10.1038/jes.2016.8. PMID 26956939.
- ↑ John Bucher; Mike Shelby. "Since you asked – Bisphenol A (BPA): Questions and Answers about Bisphenol A". National Institute of Environmental Health Sciences. Retrieved 2 February 2012.
- ↑ Staff, FDA. January 2010; Updated March 2013. Bisphenol A (BPA): Use in Food Contact Application Accessed May 7, 2013
- ↑ Staff, FDA, DRAFT version August 14, 2008 Draft Assessment of Bisphenol A for Use in Food Contact Applications Accessed May 7, 2013
- ↑ Panzica GC, Viglietti-Panzica C, Mura E, Quinn MJ, Lavoie E, Palanza P, Ottinger MA (2007). "Effects of xenoestrogens on the differentiation of behaviorally-relevant neural circuits". Frontiers in neuroendocrinology. 28 (4): 179–200. doi:10.1016/j.yfrne.2007.07.001. PMID 17868795.
- ↑ Palanza P, Gioiosa L, vom Saal FS, Parmigiani S (2008). "Effects of developmental exposure to bisphenol a on brain and behavior in mice". Environmental research. 108 (2): 150–7. Bibcode:2008ER....108..150P. doi:10.1016/j.envres.2008.07.023. PMID 18949834.
- ↑ Ogiue-Ikeda M, Tanabe N, Mukai H, Hojo Y, Murakami G, Tsurugizawa T, Takata N, Kimoto T, Kawato S (2008). "Rapid modulation of synaptic plasticity by estrogens as well as endocrine disrupters in hippocampal neurons". Brain Research Reviews. 57 (2): 363–75. doi:10.1016/j.brainresrev.2007.06.010. PMID 17822775.
- ↑ Gore AC (June 2008). "Developmental programming and endocrine disruptor effects on reproductive neuroendocrine systems". Front Neuroendocrinol. 29 (3): 358–74. doi:10.1016/j.yfrne.2008.02.002. PMC 2702520
 . PMID 18394690.
. PMID 18394690. - 1 2 Jones, DC; Miller, GW (2008). "The effects of environmental neurotoxicants on the dopaminergic system: A possible role in drug addiction". Biochemical Pharmacology. 76 (5): 569–81. doi:10.1016/j.bcp.2008.05.010. PMID 18555207.
- 1 2 "BISPHENOL A (BPA) – Current state of knowledge and future actions by WHO and FAO" (PDF). 27 November 2009. Retrieved 2 December 2009.
- ↑ Soto AM, Sonnenschein C (2010). "Environmental causes of cancer: Endocrine disruptors as carcinogens". Nature Reviews Endocrinology. 6 (7): 363–70. doi:10.1038/nrendo.2010.87. PMID 20498677.
- ↑ Bagchi, Debasis (2010). Genomics, Proteomics and Metabolomics in Nutraceuticals and Functional Foods. Wiley. p. 319. ISBN 0-8138-1402-2.
- ↑ Dolinoy DC, Huang D, Jirtle RL (2007). "Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development". Proceedings of the National Academy of Sciences. 104 (32): 13056–61. Bibcode:2007PNAS..10413056D. doi:10.1073/pnas.0703739104. PMC 1941790
 . PMID 17670942.
. PMID 17670942. - ↑ Brisken, Cathrin (2008). "Endocrine Disruptors and Breast Cancer". CHIMIA International Journal for Chemistry. 62 (5): 406–409. doi:10.2533/chimia.2008.406.
- 1 2 3 4 5 6 7 Rochester, JR (December 2013). "Bisphenol A and human health: a review of the literature". Reproductive Toxicology. 42: 132–55. doi:10.1016/j.reprotox.2013.08.008. PMID 23994667.
- ↑ Zhu H, Zheng J, Xiao X, Zheng S, Dong K, Liu J, Wang Y (2010). "Environmental endocrine disruptors promote invasion and metastasis of SK-N-SH human neuroblastoma cells". Oncology Reports. 23 (1): 129–39. doi:10.3892/or_00000614. PMID 19956873.
- 1 2 "New Study Links BPA and Childhood Asthma". Scientific American. 2013-03-01. Retrieved 2014-02-01.
- ↑ Midoro-Horiuti T, Tiwari R, Watson CS, Goldblum RM (2010). "Maternal bisphenol a exposure promotes the development of experimental asthma in mouse pups". Environmental Health Perspectives. 118 (2): 273–7. doi:10.1289/ehp.0901259. PMC 2831929
 . PMID 20123615.
. PMID 20123615. - ↑ Oaklander, M (7 October 2014). "The Link Between Asthma and This Chemical". TIME. Retrieved 20 October 2015.
- ↑ "Widely used chemical linked to childhood asthma". CBS News. Retrieved 2 March 2013.
- ↑ Dodds E. C.; Lawson Wilfrid (1936). "Synthetic Œstrogenic Agents without the Phenanthrene Nucleus". Nature. 137 (3476): 996. Bibcode:1936Natur.137..996D. doi:10.1038/137996a0.
- ↑ Rubin BS (2011). "Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects". J.Steroid Biochem.Mol.Bio. 127 (1-2): 27–34. doi:10.1016/j.jsbmb.2011.05.002. PMID 21605673.
- ↑ Gore AC (8 June 2007). Endocrine-Disrupting Chemicals: From Basic Research to Clinical Practice. Contemporary Endocrinology. Humana Press. ISBN 978-1-58829-830-0.
- ↑ O'Connor JC, Chapin RE (2003). "Critical evaluation of observed adverse effects of endocrine active substances on reproduction and development, the immune system, and the nervous system" (PDF). Pure Appl. Chem. 75 (11–12): 2099–2123. doi:10.1351/pac200375112099. Retrieved 28 February 2007.
- ↑ Okada H, Tokunaga T, Liu X, Takayanagi S, Matsushima A, Shimohigashi Y (January 2008). "Direct evidence revealing structural elements essential for the high binding ability of bisphenol A to human estrogen-related receptor-gamma". Environ. Health Perspect. 116 (1): 32–8. doi:10.1289/ehp.10587. PMC 2199305
 . PMID 18197296.
. PMID 18197296. - 1 2 vom Saal FS, Myers JP (2008). "Bisphenol A and Risk of Metabolic Disorders". JAMA. 300 (11): 1353–5. doi:10.1001/jama.300.11.1353. PMID 18799451.
- 1 2 Draft Screening Assessment for The Challenge Phenol, 4,4' -(1-methylethylidene)bis- (Bisphenol A)Chemical Abstracts Service Registry Number 80-05-7. Archived 5 September 2012 at the Wayback Machine. Health Canada, 2008.
- 1 2 Mittelstaedt, Martin (7 April 2007). "'Inherently toxic' chemical faces its future". The Globe and Mail. Toronto. Retrieved 7 April 2007.
- ↑ This table is adapted from: EWG, 2007. "Many studies confirm BPA's low-dose toxicity across a diverse range of toxic effects," Environmental Working Group Report: A Survey of Bisphenol A in U.S. Canned Foods. Retrieved 4 November 2007 at ewg.org. All studies included in this table where judged by the CERHR panel to be at least of moderate usefulness for assessing the risk of BPA to human reproduction.
- ↑ Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM (2005). "Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract". Biology of Reproduction. 72 (6): 1344–51. doi:10.1095/biolreprod.104.036301. PMID 15689538. Retrieved 1 February 2012.
- ↑ Muñoz-de-Toro M, Markey CM, Wadia PR, Luque EH, Rubin BS, Sonnenschein C, Soto AM (2005). "Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice". Endocrinology. 146 (9): 4138–47. doi:10.1210/en.2005-0340. PMC 2834307
 . PMID 15919749.
. PMID 15919749. - ↑ Newbold RR, Jefferson WN, Padilla-Banks E (2009). "Prenatal exposure to bisphenol a at environmentally relevant doses adversely affects the murine female reproductive tract later in life". Environmental Health Perspectives. 117 (6): 879–85. doi:10.1289/ehp.0800045. PMC 2702400
 . PMID 19590677.
. PMID 19590677. - ↑ Nagel SC, vom Saal FS, Thayer KA, Dhar MG, Boechler M, Welshons WV (1997). "Relative binding affinity-serum modified access (RBA-SMA) assay predicts the relative in vivo bioactivity of the xenoestrogens bisphenol A and octylphenol". Environ. Health Perspect. 105 (1): 70–6. doi:10.2307/3433065. JSTOR 3433065. PMC 1469837
 . PMID 9074884.
. PMID 9074884. - ↑ Honma S, Suzuki A, Buchanan DL, Katsu Y, Watanabe H, Iguchi T (2002). "Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction". Reproductive Toxicology. 16 (2): 117–22. doi:10.1016/S0890-6238(02)00006-0. PMID 11955942.
- ↑ Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP (2004). "Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells". Endocrinology. 145 (2): 592–603. doi:10.1210/en.2003-1174. PMID 14605012.
- ↑ Murray TJ, Maffini MV, Ucci AA, Sonnenschein C, Soto AM (2007). "Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal bisphenol A exposure". Reproductive Toxicology. 23 (3): 383–90. doi:10.1016/j.reprotox.2006.10.002. PMC 1987322
 . PMID 17123778.
. PMID 17123778. - ↑ Ho SM, Tang WY, Belmonte de Frausto J, Prins GS (2006). "Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4". Cancer Res. 66 (11): 5624–32. doi:10.1158/0008-5472.CAN-06-0516. PMC 2276876
 . PMID 16740699.
. PMID 16740699. - ↑ Palanza PL, Howdeshell KL, Parmigiani S, vom Saal FS (2002). "Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice". Environmental Health Perspectives. 110 Suppl 3 (Suppl 3): 415–22. doi:10.1289/ehp.02110s3415. PMC 1241192
 . PMID 12060838.
. PMID 12060838. - ↑ Kubo K, Arai O, Omura M, Watanabe R, Ogata R, Aou S (2003). "Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats". Journal of Neuroscience Research. 45 (3): 345–56. doi:10.1016/S0168-0102(02)00251-1. PMID 12631470.
- ↑ Leranth C, Hajszan T, Szigeti-Buck K, Bober J, MacLusky NJ (September 2008). "Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates". Proc. Natl. Acad. Sci. U.S.A. 105 (37): 14187–91. Bibcode:2008PNAS..10514187L. doi:10.1073/pnas.0806139105. PMC 2544599
 . PMID 18768812.
. PMID 18768812. - ↑ Adewale HB, Jefferson WN, Newbold RR, Patisaul HB (2009). "Neonatal bisphenol-a exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons". Biology of Reproduction. 81 (4): 690–9. doi:10.1095/biolreprod.109.078261. PMC 2754884
 . PMID 19535786.
. PMID 19535786. - ↑ Taylor JA, Welshons WV, Vom Saal FS (February 2008). "No effect of route of exposure (oral; subcutaneous injection) on plasma bisphenol A throughout 24h after administration in neonatal female mice". Reprod. Toxicol. 25 (2): 169–76. doi:10.1016/j.reprotox.2008.01.001. PMC 3564515
 . PMID 18295446. Retrieved 5 May 2008.
. PMID 18295446. Retrieved 5 May 2008. - 1 2 "Integrated Risk Information System: Bisphenol A. (CASRN 80-05-7): Oral RfD Assessment: Bisphenol A". United States Environmental Protection Agency. 1988. Retrieved 2 February 2012.
- ↑ Ryan BC, Hotchkiss AK, Crofton KM, Gray LE (2010). "In utero and lactational exposure to bisphenol A, in contrast to ethinyl estradiol, does not alter sexually dimorphic behavior, puberty, fertility, and anatomy of female LE rats". Toxicological Sciences. 114 (1): 133–48. doi:10.1093/toxsci/kfp266. PMID 19864446.
- ↑ Sharpe RM (2010). "Is it time to end concerns over the estrogenic effects of bisphenol A?". Toxicological Sciences. 114 (1): 1–4. doi:10.1093/toxsci/kfp299. PMID 20147444.
- ↑ vom Saal FS, Akingbemi BT, Belcher SM, Crain DA, Crews D, Guidice LC, Hunt PA, Leranth C, Myers JP, Nadal A, Olea N, Padmanabhan V, Rosenfeld CS, Schneyer A, Schoenfelder G, Sonnenschein C, Soto AM, Stahlhut RW, Swan SH, Vandenberg LN, Wang HS, Watson CS, Welshons WV, Zoeller RT (2010). "Flawed Experimental Design Reveals the Need for Guidelines Requiring Appropriate Positive Controls in Endocrine Disruption Research". Toxicological Sciences. 115 (2): 612–3; author reply 614–20. doi:10.1093/toxsci/kfq048. PMID 20164146.
- ↑ Gray, L. E. (2010). "Rebuttal of "Flawed Experimental Design Reveals the Need for Guidelines Requiring Appropriate Positive Controls in Endocrine Disruption Research" by vom Saal". Toxicological Sciences. 115 (2): 614–620. doi:10.1093/toxsci/kfq073.
- 1 2 Erler C, Novak J (October 2010). "Bisphenol a exposure: human risk and health policy". J Pediatr Nurs. 25 (5): 400–7. doi:10.1016/j.pedn.2009.05.006. PMID 20816563.
- ↑ EPA (July 26, 2011). "Testing of Bisphenol A, Advance notice of proposed rulemaking (ANPRM)". Federal Register /Vol. 76, No. 143 / Proposed Rules. Federal Register.
- ↑ Barry, Carolyn (20 August 2009). "Plastic Breaks Down in Ocean, After All – And Fast". National Geographic News. Retrieved 1 February 2012.
- ↑ Fox JE, Gulledge J, Engelhaupt E, Burow ME, McLachlan JA (2007). "Pesticides reduce symbiotic efficiency of nitrogen-fixing rhizobia and host plants". Proc. Natl. Acad. Sci. 104 (24): 10282–7. Bibcode:2007PNAS..10410282F. doi:10.1073/pnas.0611710104. PMC 1885820
 . PMID 17548832.
. PMID 17548832. - ↑ Drewes, J. E.; Hemming, J.; Ladenburger, S. J.; Schauer, J.; Sonzogni, W. An assessment of endocrine disrupting activity changes during wastewater treatment through the use of bioassays and chemical measurements. Water Environ. Res. 2005, 77, 12–23.
- ↑ Klečka, G., Staples, C., Clark, K., Anderhoeven, N., Thomas, D. and Hentges, S. (2009). "Exposure analysis of Bisphenol A in surface water systems in North America and Europe". Environ. Sci. Technol. 43: 6145–6150. Bibcode:2009EnST...43.6145K. doi:10.1021/es900598e.
- ↑ "Bisphenol A Fact Sheet". Government of Canada. Archived from the original on 23 April 2011. Retrieved 1 February 2012.
- ↑ Oehlmann J, Schulte-Oehlmann U, Kloas W, Jagnytsch O, Lutz I, Kusk KO, Wollenberger L, Santos EM, Paull GC, Van Look KJ, Tyler CR (2009). "A critical analysis of the biological impacts of plasticizers on wildlife". Philosophical Transactions of the Royal Society B: Biological Sciences. 364 (1526): 2047–62. doi:10.1098/rstb.2008.0242. PMC 2873012
 . PMID 19528055.
. PMID 19528055. - ↑ Brown, Eryn. "Jury still out on BPA, World Health Organization says", The Los Angeles Times, 11 November 2010. Retrieved 7 February 2011.
- ↑ "Toxicological and Health Aspects of Bisphenol-A". who.int. 2011.
- ↑ "Questions & Answers on Bisphenol A (BPA) Use in Food Contact Applications". Fda.gov. 2013-06-04. Retrieved 2014-02-01.
- ↑ "Final Report for the Review of Literature and Data on BPA" (PDF). Fda.gov. 2014-06-06.
- ↑ "Bisphenol A (BPA) and food packaging". Food Standards Australia New Zealand. May 2009. Archived from the original on 9 January 2010. Retrieved 1 February 2012.
- ↑ "Babies' bottles and bisphenol A". New Zealand Food Safety Authority. May 2008. Archived from the original on 26 July 2010. Retrieved 1 February 2012.
- ↑ "Bisphenol A (BPA)". Food Standards Australia New Zealand. April 2012.
- ↑ Morrissey, Susan R. (23 April 2008). "Banning Bisphenol A In Baby Bottles: Canada moves toward restricting the chemical; Congress proposes similar legislation". Chemical and Engineering News.
- ↑ "Bisphenol A". Health Canada. Retrieved 2 February 2012.
- ↑ Canoe.ca, Politics: Bisphenol A water-bottle removal expanding among Canadian retailers. Archived 6 June 2008 at the Wayback Machine.
- ↑ "Government of Canada Protects Families With Bisphenol A Regulations". Health Canada. 17 October 2008. Archived from the original on 26 February 2010. Retrieved 1 February 2012.
- ↑ "Order Adding a Toxic Substance to Schedule 1 to the Canadian Environmental Protection Act, 1999". Canada Gazette. 23 September 2010. Retrieved 2 February 2012.
- ↑ Rapporteur: United Kingdom. (2008). JRC.it Updated European Risk Assessment Report: 4,4'-ISOPROPYLIDENEDIPHENOL (BISPHENOL-A) Archived 15 February 2010 at the Wayback Machine. FINAL APPROVED VERSION AWAITING FOR PUBLICATION. EFSA, Press release
- ↑ http://arquivo.pt/wayback/wayback/20091008003630/http%3A//www%2Eefsa%2Eeuropa%2Eeu/EFSA/efsa_locale%2D1178620753812_1211902145465%2Ehtm. Archived from the original on 8 October 2009. Retrieved 25 May 2009. Missing or empty
|title=(help) - ↑ "Council conclusions on combination effects of chemicals". Brussels: Council of The European Union. 22 December 2009. Retrieved 30 December 2009.
- 1 2 "Scientific Opinion on Bisphenol A: evaluation of a study investigating its neurodevelopmental toxicity, review of recent scientific literature on its toxicity and advice on the Danish risk assessment of Bisphenol A". European Food Safety Authority. 23 September 2010. Retrieved 23 September 2010.
- ↑ "EU to ban Bisphenol A in baby bottles in 2011". Reuters. 25 November 2010. Retrieved 2 February 2012.
- ↑ "Europe bans baby bottles with Bisphenol-A". PhysOrg.com. 25 November 2010. Retrieved 2 February 2012.
- ↑ "EU bans bisphenol A chemical from babies' bottles". BBC News. 25 November 2010.
I do not know of any convincing evidence that bisphenol A exposure, in the amounts used in polycarbonate bottles, can cause any harm to babies, as not only are the amounts so minuscule but they are rapidly broken down in the gut and liver.
- 1 2 "Umstrittene Chemikalie: EU-Behörde senkt Grenzwert für Bisphenol A" (in German). Der Spiegel. January 21, 2015. Retrieved January 21, 2015.
- ↑ "Bisphenol A".
- ↑ "EFSA Topic: Bisphenol A". European Safety Authority. October 24, 2014. Retrieved October 27, 2014.
- ↑ "Danmark forbyder giftigt stof i sutteflasker" (in Danish). DR. 26 March 2010. Retrieved 31 January 2012.
- ↑ "Negen op tien zuigflessen zijn gevaarlijk" (in Dutch). Microsoft. 3 May 2010. Retrieved 31 January 2012.
- ↑ http://www.tssbelgium.be/printversion/senate.5.1037.1.pdf
- ↑ "Bisphenol A: AFSSA recommends the development of new assessment methods". 5 February 2010. Retrieved 8 February 2010.
- ↑ "Opinion of the French Food Safety Agency on the critical analysis of the results of a study of the toxicity of bisphenol A on the development of the nervous system together with other recently-published data on its toxic effects" (PDF). French Food Safety Agency. 29 January 2010. Retrieved 8 February 2010.
- ↑ "Afssa: informations sur le Bisphénol A". Le Figaro (in French). 27 April 2010. Retrieved 7 May 2010.
- ↑ "Les sénateurs votent la suspension de la commercialisation des biberons au Bisphenol A". Le Monde (in French). 24 March 2010. Retrieved 24 March 2010.
- ↑ "Détail d'un texte" (in French). Legifrance.gouv.fr. Retrieved 23 October 2011.
- ↑ "L'Assemblée unanime interdit les contenants alimentaires avec du bisphénol A – Libération". Libération (in French). Retrieved 23 October 2011.
- ↑ "Le Sénat a adopté une proposition de loi visant au retrait des conditionnements alimentaires en bisphénol A – Sénat". Sénat (in French). Retrieved 31 October 2012.
- ↑ "Neue Studien zu Bisphenol A stellen die bisherige Risikobewertung nicht in Frage" (PDF). Bundesinstitut für Risikobewertung (in German). 19 September 2008. Retrieved 2 February 2012.
- ↑ "Schnuller geben hormonell wirksames Bisphenol A ab. BUND fordert Verbot der Chemikalie für Babyartikel" (in German). Retrieved 15 October 2009.
- ↑ "Chemikalie in Schnullern entdeckt" (in German). 1 October 2009. Retrieved 2 October 2009.
- ↑ "Bisphenol-A: Kehrtwende". MedizinAuskunft (in German). 4 November 2009. Archived from the original on 19 July 2011. Retrieved 1 February 2012.
- ↑ http://www.vwa.nl/cdlpub/servlet/CDLServlet?p_file_id=31842. Retrieved 25 May 2009. Missing or empty
|title=(help) - ↑ "Bisphenol A". Bundesamt für Gesundheit. Retrieved 1 February 2012.
- ↑ "Minister kör över Livsmedelsverket" (in Swedish). Dagens Handel. 11 May 2010. Retrieved 1 February 2012.
- ↑ BioNut researcher on ban of bisphenol A (BPA) Archived 30 September 2011 at the Wayback Machine. 10 May 2010. accessdate 13 May 2010.
- ↑ "Sweden acts on endocrine disruptor Bisphenol A". ChemSec. Retrieved 16 April 2012.
- ↑ Smith, Rebecca (1 December 2009). "Ban chemical linked to cancer in baby bottles: campaigners". The Daily Telegraph. Retrieved 16 February 2010.
- ↑ "US Acts on BPA and Baby Bottles while UK Food Standards Agency dismisses concerns". Breast Cancer UK. 18 January 2009. Retrieved 16 February 2010.
- ↑ "Başbakanlık Mevzuatı Geliştirme ve Yayın Genel Müdürlüğü" (in Turkish). Resmigazete.gov.tr. Retrieved 23 October 2011.
- ↑ "Bisphenol A: Toxic Plastics Chemical in Canned Food: Companies reduced BPA exposures in Japan". Environmental Working Group. Retrieved 3 February 2012.
- ↑ Cichna-Markl, M. Methods (2012).
- ↑ "A Survey of Bisphenol A in U.S. Canned Foods". Environmental Working Group. 5 March 2007. Retrieved 2 February 2012.
- ↑ "Pipeline relining". Retrieved 18 October 2010.
- 1 2 TSAI, WEN-TIEN (2006-12-01). "Human Health Risk on Environmental Exposure to Bisphenol-A: A Review". Journal of Environmental Science and Health, Part C. 24 (2): 225–255. doi:10.1080/10590500600936482. ISSN 1059-0501.
- ↑ "What Is BPA? - Bisphenol A (BPA) FAQs – Frequently Asked Questions". bisphenol-a.org.
- ↑ "Significant bisphenol A levels found in canned food". 4 November 2009. Retrieved 4 November 2009.
- ↑ Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL (2005). "Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population". Environ. Health Perspect. 113 (4): 391–5. doi:10.1289/ehp.7534. PMC 1278476
 . PMID 15811827.
. PMID 15811827. - ↑ Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL (2008). "Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004". Environ. Health Perspect. 116 (1): 39–44. doi:10.1289/ehp.10753. PMC 2199288
 . PMID 18197297.
. PMID 18197297. - 1 2 3 Lakind JS, Naiman DQ (2010). "Daily intake of bisphenol a and potential sources of exposure: 2005–2006 National Health and Nutrition Examination Survey". Journal of Exposure Science and Environmental Epidemiology. 21 (3): 272–9. doi:10.1038/jes.2010.9. PMC 3079892
 . PMID 20237498.
. PMID 20237498. - ↑ Health Canada. "Survey of Bisphenol A in Canned Drink Products". Retrieved 13 March 2009.
- ↑ Schecter A, Malik N, Haffner D, Smith S, Harris TR, Paepke O, Birnbaum L (2010). "Bisphenol A (BPA) in U.S. Food". Environmental Science & Technology. 44 (24): 9425–30. Bibcode:2010EnST...44.9425S. doi:10.1021/es102785d. PMID 21038926.
- ↑ "Food Packaging and Bisphenol A and Bis(2-Ethyhexyl) Phthalate Exposure: Findings from a Dietary Intervention". Journalist's Resource.org.
- ↑ Taylor, Paul (24 November 2011). ""BPA being absorbed from canned food" at theglobeandmail.com". The Globe and Mail. Canada.
- ↑ Carwile JL, Luu HT, Bassett LS, Driscoll DA, Yuan C, Chang JY, Ye X, Calafat AM, Michels KB (2009). "Polycarbonate Bottle Use and Urinary Bisphenol A Concentrations". Environ. Health Perspect. 117 (9): 1368–72. doi:10.1289/ehp.0900604. PMC 2737011
 . PMID 19750099.
. PMID 19750099. - ↑ Ashley Ahearn (19 September 2008). "War of the Sciences". Living on Earth. Retrieved 2 February 2012.
- ↑ "FDA Weighs Safety Of Bisphenol A". NPR. Retrieved 23 October 2011.
- ↑ Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D (2008). "Association of Urinary Bisphenol A Concentration With Medical Disorders and Laboratory Abnormalities in Adults". Journal of the American Medical Association. 300 (11): 1303–10. doi:10.1001/jama.300.11.1303. PMID 18799442.
- ↑ Fukazawa H, Hoshino K, Shiozawa T, Matsushita H, Terao Y (2001). "Identification and quantification of chlorinated bisphenol a in wastewater from wastepaper recycling plants". Chemosphere. 44 (5): 973–9. doi:10.1016/S0045-6535(00)00507-5. PMID 11513431.
- ↑ Raloff, Janet (7 October 2009). "Concerned About BPA: Check Your Receipts". Society for Science and the Public. Retrieved 7 October 2009.
- ↑ Gehring M, Tennhardt L, Vogel D, Weltin D, Bilitewski B (2004). "Bisphenol A Contamination of Wastepaper, Cellulose and Recycled Paper Products" (PDF). Transactions on Ecology and the Environment. 78: 294–300. Lay summary (PDF).
- 1 2 Babu, S.; Uppu, S. N.; Martin, B.; Agu, O. A.; Uppu, R. M. (2015). "Unusually high levels of bisphenol A (BPA) in thermal paper cash register receipts (CRs): development and application of a robust LC-UV method to quantify BPA in CRs.". Toxicology mechanisms and methods. 25: 410–6. doi:10.3109/15376516.2015.1045661. PMID 26024012.
- ↑ Biedermann S, Tschudin P, Grob K (September 2010). "Transfer of bisphenol A from thermal printer paper to the skin". Anal Bioanal Chem. 398 (1): 571–6. doi:10.1007/s00216-010-3936-9. PMID 20623271.
- ↑ Liao C, Kannan K (August 2011). "High levels of bisphenol A in paper currencies from several countries, and implications for dermal exposure". Environ. Sci. Technol. 45 (16): 6761–8. Bibcode:2011EnST...45.6761L. doi:10.1021/es200977t. PMID 21744851.
- ↑ Liao C, Kannan K (November 2011). "Widespread occurrence of bisphenol A in paper and paper products: implications for human exposure". Environ. Sci. Technol. 45 (21): 9372–9. Bibcode:2011EnST...45.9372L. doi:10.1021/es202507f. PMID 21939283.
- ↑ "Study: Americans have twice as much BPA as Canadians". USA Today. 3 March 2011.
- ↑ Vandenberg LN (2011) Exposure to bisphenol A in Canada: invoking the precautionary principle. CMAJ (183)11:1265–1270 doi:10.1503/cmaj.101408 PMID 21343266
- ↑ LaKind, Judy S.; Naiman, Daniel Q. (2015-10-01). "Temporal trends in bisphenol A exposure in the United States from 2003–2012 and factors associated with BPA exposure: Spot samples and urine dilution complicate data interpretation". Environmental Research. 142: 84–95. doi:10.1016/j.envres.2015.06.013.
- ↑ Diamanti-Kandarakis, E (2009). "Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocrine Reviews".
- ↑ Edginton AN, Ritter L (2009). "Predicting plasma concentrations of bisphenol a in children younger than 2 years of age after typical feeding schedules, using a physiologically based toxicokinetic model". Environmental Health Perspectives. 117 (4): 645–52. doi:10.1289/ehp.0800073. PMC 2679611
 . PMID 19440506.
. PMID 19440506. - ↑ Beronius A, Rudén C, Håkansson H, Hanberg A (April 2010). "Risk to all or none? A comparative analysis of controversies in the health risk assessment of Bisphenol A". Reprod. Toxicol. 29 (2): 132–46. doi:10.1016/j.reprotox.2009.11.007. PMID 19931376.
- ↑ "European Food Safety Authority Opinion" (Abstract). Retrieved 28 February 2007.
- 1 2 Ackerman LK, Noonan GO, Heiserman WM, Roach JA, Limm W, Begley TH (2010). "Determination of bisphenol a in U.S. Infant formulas: Updated methods and concentrations". Journal of Agricultural and Food Chemistry. 58 (4): 2307–13. doi:10.1021/jf903959u. PMID 20102208.
- ↑ Cao XL, Dufresne G, Belisle S, Clement G, Falicki M, Beraldin F, Rulibikiye A (2008). "Levels of bisphenol a in canned liquid infant formula products in Canada and dietary intake estimates". Journal of Agricultural and Food Chemistry. 56 (17): 7919–24. doi:10.1021/jf8008712. PMID 18702469.
- ↑ "Bisphenol A (BPA) Information for Parents". U.S. Department of Health and Human Services. Retrieved 25 March 2011.
- ↑ Zimmers, Stephanie M.; Browne, Eva P.; O'Keefe, Patrick W.; Anderton, Douglas L.; Kramer, Lawrence; Reckhow, David A.; Arcaro, Kathleen F. (2014-06-01). "Determination of free Bisphenol A (BPA) concentrations in breast milk of U.S. women using a sensitive LC/MS/MS method". Chemosphere. 104: 237–243. doi:10.1016/j.chemosphere.2013.12.085. ISSN 1879-1298. PMID 24507723.
- ↑ Mendonca, K.; Hauser, R.; Calafat, A. M.; Arbuckle, T. E.; Duty, S. M. (2014-01-01). "Bisphenol A concentrations in maternal breast milk and infant urine". International Archives of Occupational and Environmental Health. 87 (1): 13–20. doi:10.1007/s00420-012-0834-9. ISSN 1432-1246. PMC 4381877
 . PMID 23212895.
. PMID 23212895. - ↑ von Goetz N, Wormuth M, Scheringer M, Hungerbühler K (2010). "Bisphenol A: How the Most Relevant Exposure Sources Contribute to Total Consumer Exposure". Risk Analysis. 30 (3): 473–87. doi:10.1111/j.1539-6924.2009.01345.x. PMID 20136739.
- ↑ "Bisphenol A (BPA): Use in Food Contact Application". US Food and Drug Administration. January 2015. Retrieved 25 October 2015.
- ↑ Zalko D, Soto AM, Dolo L, Dorio C, Rathahao E, Debrauwer L, Faure R, Cravedi JP (2003). "Biotransformations of bisphenol a in a mammalian model: Answers and new questions raised by low-dose metabolic fate studies in pregnant CD1 mice". Environmental Health Perspectives. 111 (3): 309–19. doi:10.1289/ehp.5603. PMC 1241388
 . PMID 12611660.
. PMID 12611660. - ↑ Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, Lanphear BP (2009). "Prenatal Bisphenol a Exposure and Early Childhood Behavior". Environmental Health Perspectives. 117 (12): 1945–52. doi:10.1289/ehp.0900979. PMC 2799471
 . PMID 20049216.
. PMID 20049216. - ↑ "Cord Blood Contaminants in Minority Newborns" (PDF). Environmental Working Group. 2009. Retrieved 2 December 2009.
- ↑ "BPA Exposure in Pregnancy May Affect Behavior in Girls – TIME.com". Time. 24 October 2011.
- ↑ Sun Y, Irie M, Kishikawa N, Wada M, Kuroda N, Nakashima K (2004). "Determination of bisphenol a in human breast milk by HPLC with column-switching and fluorescence detection". Biomedical chromatography : BMC. 18 (8): 501–7. doi:10.1002/bmc.345. PMID 15386523.

- ↑ Ye X, Kuklenyik Z, Needham LL, Calafat AM (2006). "Measuring environmental phenols and chlorinated organic chemicals in breast milk using automated on-line column-switching-high performance liquid chromatography-isotope dilution tandem mass spectrometry". Journal of Chromatography B. 831 (1–2): 110–5. doi:10.1016/j.jchromb.2005.11.050. PMID 16377264.
- ↑ Sajiki J, Yanagibori R, Kobayashi Y (2010). "Study of experiment on leaching of bisphenol a from infant books to artificial saliva". Nihon eiseigaku zasshi. Japanese journal of hygiene. 65 (3): 467–70. doi:10.1265/jjh.65.467. PMID 20508389.
- ↑ Charles Schumer; Dianne Feinstein; Hillary Clinton; Richard Durbin; John Kerry; Robert Menendez) (April 29, 2008). "Bill Text 110th Congress (2007–2008) S.2928.IS". Thomas. Library of Congress. Retrieved 23 March 2014.
- 1 2 Layton, Lindsey (16 September 2008). "Study Links Chemical BPA to Health Problems". The Washington Post. pp. A03. Retrieved 17 September 2008.
- ↑ Szabo, Liz. "Scientists, FDA face off over safety of BPA in consumer plastics". USA Today. 17 September 2008. Retrieved 29 October 2008
- ↑ National Research Center for Women and Families (16 September 2008). "Statement of Diana Zuckerman, PhD At the FDA Public Meeting on its Draft Assessment of Bisphenol A for Use in Food Contact Applications". Retrieved 15 July 2009.
- ↑ Karen Forman (5 March 2009). "County bans baby bottle plastic with BPA". northshoreoflongisland.com. Retrieved 2 February 2012.
- ↑ "Bills Would Ban BPA From Food and Drink Containers". The Washington Post. 14 March 2009. Retrieved 2 February 2012.
- ↑ Meg Kissinger; Susanne Rust (11 April 2009). "Scientists reject FDA assertion of BPA's safety". Contra Costa Times. Archived from the original on 16 April 2009. Retrieved 2 February 2012.
- ↑ Sternberg, Bob von (8 May 2009). "State bans chemical in baby bottles". Star Tribune. Retrieved 11 July 2009.
- ↑ "Chicago BPA ban: Chicago bans sale of baby bottles, sippy cups with dangerous chemical … linked to diabetes, cancer and other illnesses". Chicago Tribune. 14 May 2009. Archived from the original on 24 March 2010. Retrieved 1 February 2012.
- ↑ Favole, Jared A. (3 June 2009). "FDA to Revisit Decision on Safety of BPA". The Wall Street Journal. Retrieved 23 October 2011.
- ↑ Bodach, Jill (5 June 2009). "Connecticut first state to ban BPA". The Hour. The Hour Publishing Co. Retrieved 9 September 2009.
- ↑ "Calif. regulators will not list Bisphenol A under Prop. 65, call for more study". Los Angeles Times. Retrieved 19 July 2009.
- ↑ "California won't warn public about bisphenol A". Los Angeles Times. 17 July 2009. Retrieved 23 October 2011.
- ↑ "Existing Chemical Action Plans". Retrieved 1 October 2009.
- ↑ Sarewitz D (December 2009). "World view: A tale of two sciences". Nature. 462 (7273): 566. doi:10.1038/462566a. PMID 19956236.
- ↑ "Update on Bisphenol A for Use in Food Contact Applications: January 2010" (PDF). U.S. Food and Drug Administration. 15 January 2010. Retrieved 15 January 2010.
- ↑ "Bisphenol A (BPA) Information for Parents". U.S. Department of Health & Human Services. 15 January 2010. Retrieved 15 January 2010.
- ↑ Koch, Wendy (16 February 2010). "Bans sought for chemical BPA in baby, toddler products". USA Today. Retrieved 17 February 2010.
- ↑ Reuben, Suzanne H. (April 2010). "Annual Report for 2008–2009" (PDF). National Cancer Institute. Archived from the original (PDF) on 2 June 2010. Retrieved 1 February 2012.
- ↑ "Maine board weighs BPA bottle ban – Maine Politics – Bangor Daily News". New.bangordailynews.com. 18 June 2010. Archived from the original on 23 December 2010. Retrieved 23 October 2011.
- ↑ Kevin Miller (22 February 2011). "LePage dismisses BPA dangers; 'worst case is some women may have little beards'". Health & Fitness – Bangor Daily News. Retrieved 2 February 2012.
- ↑ "Maine Gov. Paul LePage On BPA: 'Worst Case Is Some Women May Have Little Beards'". Huffington Post. 23 February 2011.
- ↑ "BPA Phase-Out Becomes Maine Law". Good Chemistry. 25 April 2011. Retrieved 31 January 2012.
- ↑ "Greenspace". Los Angeles Times. 5 October 2011.
- ↑ Shader, Maggie (6 October 2011). "California Joins 10 Other States in Banning BPA From Infant Feeding Containers". Consumer News. Consumerreports.org.
- ↑ American Medical Association (4 July 2011). "AMA supports tighter restrictions on products containing BPA".
- ↑ "FDA Continues to Study BPA". FDA Consumer Updates. March 2012. Retrieved 4 April 2012.
- ↑ "BPA Banned From Baby Bottles". Huffington Post. 17 July 2012.
- ↑ Sabrina Tavernise (July 17, 2012). "F.D.A. Makes It Official: BPA Can't Be Used in Baby Bottles and Cups". NYTimes.
- ↑ "About Bisphenol A". Safer States. January 24, 2013. Retrieved 23 March 2014.
- ↑ Kissinger, Meg (14 February 2010). "Regulator waffles on bisphenol A". The Milwaukee Journal Sentinel. Retrieved 17 February 2010.
- ↑ "EPA delays action on suspect chemical". United Press International. 14 February 2010. Retrieved 17 February 2010.
- ↑ "EPA to Scrutinize Environmental Impact of Bisphenol A". US EPA. 29 March 2010. Retrieved 12 April 2010.
- ↑ US EPA (July 26, 2011). "Testing of Bisphenol A". Federal Register/Vol. 76, No. 143.
- ↑ Carroll, Jeremy (20 September 2013). "EPA withdraws draft rules on BPA, PBDEs". Plastics News. Crain Communications Inc. Retrieved 13 March 2014.
- ↑ Denison, Richard (September 6, 2013). "Stymied at every turn: EPA withdraws two draft TSCA proposals in the face of endless delay at OMB". EDF Health. EDF. Retrieved 23 March 2014.
- ↑ "BPA Alternatives in Thermal Paper Partnership" (PDF). Design for Environment. EPA. 29 January 2014. Retrieved 23 March 2014.
- ↑ Lyndsey Layton (6 March 2009). "No BPA For Baby Bottles In U.S.". The Washington Post. Retrieved 2 February 2012.
- ↑ Matthew Perrone (12 March 2009). "Sunoco restricts sales of chemical used in bottles". The Seattle Times. Retrieved 3 February 2012.
- ↑ Layton, Lyndsey (31 May 2009). "Strategy Being Devised To Protect Use of BPA". The Washington Post. Retrieved 23 October 2011.
- ↑ Meg Kissinger; Susanne Rust (22 August 2009). "BPA industry fights back". Milwaukee Journal Sentinel. Retrieved 2 February 2012.
- ↑ Susanne Rust; Meg Kissinger (22 August 2009). "'Watchdog' advocates for BPA". Milwaukee Journal Sentinel. Retrieved 2 February 2012.
- ↑ "General Mills to Pull BPA from Organic Tomato Cans, GreenerDesign". Greenbiz.com. 19 April 2010. Retrieved 23 October 2011.
- 1 2 3 "Corporate Positions on BPA". Breast CancerFund. Retrieved 21 March 2014.
- ↑ Liao C, Liu F, Kannan K (May 16, 2012). "Bisphenol S, a New Bisphenol Analogue, in Paper Products and Currency Bills and Its Association with Bisphenol A Residues". Environ. Sci. Technol. 46 (12): 6515–6522. Bibcode:2012EnST...46.6515L. doi:10.1021/es300876n. PMID 22591511. Retrieved 21 March 2014.
- 1 2 Bittner, George. "Estrogenic chemicals often leach from BPA-free plastic products that are replacements for BPA-containing polycarbonate products.". Environmental Health. doi:10.1186/1476-069x-13-41.
- ↑ Rochester, Johanna R.; Bolden, Ashley L. (5 March 2015). "Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes" (PDF). National Institute of Environmental Health Sciences. Retrieved 8 May 2015.
- ↑ Mayu Enya; Keiko Aoyagi; Yoshihiro Hishikawa; Azusa Yoshimura; Koichi Mitsukura; Kiyofumi Marayuma (2012). "Molecular and Catalytic Properties of 2,4′-Dihydroxyacetophenone Dioxygenase from Burkholderia sp. AZ11". Bioscience, Biotechnology, and Biochemistry. 76 (3): 567–574. doi:10.1271/bbb.110867.
- ↑ Prasad Kotharu; Lynda Ellis; Shawn Balcome. "Bisphenol A Pathway Map". Biocatalysis/Biodegradation Database. Retrieved 2015-11-04.
- ↑ Keum YS, Lee HR, Park HW, Kim JH (2010). "Biodegradation of bisphenol a and its halogenated analogues by Cunninghamella elegans ATCC36112". Biodegradation. 21 (6): 989–997. doi:10.1007/s10532-010-9358-8. PMID 20455075.
- ↑ Watanabe I. Harada K. Matsui T. Miyasaka H. Okuhata H. Tanaka S. Nakayama H. Kato K. Bamba T. Hirata K."Characterization of bisphenol A metabolites produced by Portulaca oleracea cv. by liquid chromatography coupled with tandem mass spectrometry." , Biotechnology & Biochemistry. 76(5):1015-7, 2012.
Further reading
- Kabiersch G, Rajasärkkä J, Ullrich R, Tuomela M, Hofrichter M, Virta M, Hatakka A, Steffen K (April 2011). "Fate of bisphenol A during treatment with the litter-decomposing fungi Stropharia rugosoannulata and Stropharia coronilla". Chemosphere. 83 (3): 226–32. doi:10.1016/j.chemosphere.2010.12.094. PMID 21295326.
- Myers JP, vom Saal FS, Akingbemi BT, Arizono K, Belcher S, Colborn T, Chahoud I, Crain DA, Farabollini F, Guillette LJ, Hassold T, Ho SM, Hunt PA, Iguchi T, Jobling S, Kanno J, Laufer H, Marcus M, McLachlan JA, Nadal A, Oehlmann J, Olea N, Palanza P, Parmigiani S, Rubin BS, Schoenfelder G, Sonnenschein C, Soto AM, Talsness CE, Taylor JA, Vandenberg LN, Vandenbergh JG, Vogel S, Watson CS, Welshons WV, Zoeller RT (March 2009). "Why public health agencies cannot depend on good laboratory practices as a criterion for selecting data: the case of bisphenol A". Environ. Health Perspect. 117 (3): 309–15. doi:10.1289/ehp.0800173. PMC 2661896
 . PMID 19337501.
. PMID 19337501.
External links
| Look up bisphenol a in Wiktionary, the free dictionary. |
| Wikimedia Commons has media related to |
- International Chemical Safety Cards – Bisphenol A NIOSH, partly updated in October 2005, retrieved 8 April 2015
- Bisphenol A (BPA) in food FDA, November 2014, retrieved 8 April 2015
- BPA Data Gap Analysis FDA, undated, retrieved 8 April 2015
- Bisphenol A website Polycarbonate/BPA Global Group, American Chemistry Council, retrieved 8 April 2015
- Bisphenol A articles Environmental Health Perspectives by NIEHS, retrieved 8 April 2015 (200 articles)(open access)
- Bisphenol A ChemSub Online, retrieved 8 April 2015
- How to Protect Your Baby from BPA (Bisphenol A) Brochure, 2 pages, 2009, Massachusetts Department of Public Health, retrieved 8 April 2015
- Plastic Not Fantastic with Bisphenol A February 19, 2008 By David Biello, www.scientificamerican.com, retrieved 8 April 2015
- Report on BPA Endocrine/Estrogen Letter Vol. 9, No. 2&3 (174¯) 2003, 31 pages, retrieved 8 April 2015
- Hazard in a bottle. The plastics industry’s Pyrrhic victory Attempt to regulate BPA in California defeated, The Economist, 22 August 2008
- Alternatives to BPA containers not easy for U.S. foodmakers to find Lyndsey Layton. Washington Post, 23 February 2010. retrieved 8 April 2015
- Is It Time to End Concerns over the Estrogenic Effects of Bisphenol A? Richard Sharpe, Toxicological Sciences (2010) 114 (1): 1-4. doi: 10.1093/toxsci/kfp299 (open access)retrieved 8 April 2015
- Hand sanitizer may increase BPA absorption in Science Friday, October 24, 2014.
- Bisphenol-A News & Products News commentary on BPA Containing Products
- bisphenol A US FDA statement, 2008
- News Story about Canadian Study on BPA Free Marketed Bottles not being BPA Free gforceproducts.com, July 30, 2009