Quisqualamine
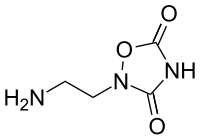 | |
| Names | |
|---|---|
| IUPAC name
2-(2-aminoethyl)-1,2,4-oxadiazolidine-3,5-dione | |
| Identifiers | |
| 68373-11-5 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 2342291 |
| PubChem | 3085372 |
| |
| |
| Properties | |
| C4H7N3O3 | |
| Molar mass | 145.12 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Quisqualamine is the α-decarboxylated analogue of quisqualic acid, as well as a relative of the neurotransmitters glutamate and γ-aminobutyric acid (GABA).[1][2] α-Decarboxylation of excitatory amino acids can produce derivatives with inhibitory effects.[2] Indeed, unlike quisqualic acid, quisqualamine has central depressant and neuroprotective properties and appears to act predominantly as an agonist of the GABAA receptor and also to a lesser extent as an agonist of the glycine receptor, due to the facts that its actions are inhibited in vitro by GABAA antagonists like bicuculline and picrotoxin and by the glycine antagonist strychnine, respectively.[1][2][3] Mg2+ and DL-AP5, NMDA receptor blockers, CNQX, an antagonist of both the AMPA and kainate receptors, and 2-hydroxysaclofen, a GABAB receptor antagonist, do not affect quisqualamine's actions in vitro, suggesting that it does not directly affect the ionotropic glutamate receptors or the GABAB receptor in any way.[2] Whether it binds to and acts upon any of the metabotropic glutamate receptors like its analogue quisqualic acid however is unclear.
See also
References
- 1 2 Evans RH, Francis AA, Hunt K, Martin MR, Watkins JC (June 1978). "Quisqualamine, a novel gamma-aminobutyric acid (GABA) related depressant amino acid". The Journal of Pharmacy and Pharmacology. 30 (6): 364–7. doi:10.1111/j.2042-7158.1978.tb13257.x. PMID 26767.
- 1 2 3 4 Herrero JF (March 1994). "GABAergic activity of quisqualamine and homoquisqualamine in hemisected spinal cord in vitro preparation". Revista Española De Fisiología. 50 (1): 11–7. PMID 7527570.
- ↑ Biraboneye AC, Madonna S, Maher P, Kraus JL (January 2010). "Neuroprotective effects of N-alkyl-1,2,4-oxadiazolidine-3,5-diones and their corresponding synthetic intermediates N-alkylhydroxylamines and N-1-alkyl-3-carbonyl-1-hydroxyureas against in vitro cerebral ischemia". Chemmedchem. 5 (1): 79–85. doi:10.1002/cmdc.200900418. PMID 19943277.