Fosfestrol
 | |
| Clinical data | |
|---|---|
| Trade names | Honvan |
| AHFS/Drugs.com | International Drug Names |
| ATC code | L02AA04 (WHO) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
13425-53-1 |
| PubChem (CID) | 3032325 |
| ChemSpider |
2297327 |
| UNII |
A0E0NMA80F |
| KEGG |
D00946 |
| ChEMBL |
CHEMBL1200598 |
| ECHA InfoCard | 100.007.573 |
| Chemical and physical data | |
| Formula | C18H22O8P2 |
| Molar mass | 428.310 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
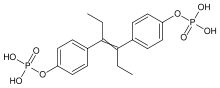
Fosfestrol (INN, BAN, JAN) (brand names Honvan, Difostilben, Fosfostilben, Fostrolin, Stilbol, Stilphostrol, Vagestrol, among others), also known as diethylstilbestrol diphosphate (USAN), abbreviated as DESDP or DESP, is a synthetic, non-steroidal estrogen of the stilbestrol group that is or has been used in the treatment of prostate cancer.[1][2] It is a prodrug of diethylstilbestrol.[3] The drug has been marketed widely throughout the world, including in the United States, Canada, Europe, Hong Kong, and Mexico.[1]
See also
References
- 1 2 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 332–. ISBN 978-3-88763-075-1.
- ↑ Shanbhag (1 January 2008). Pharmacology: Prep Manual for Undergraduates. Elsevier India. pp. 463–. ISBN 978-81-312-1153-3.
- ↑ Urotext (1 January 2001). Urotext-Luts: Urology. Urotext. pp. 386–. ISBN 978-1-903737-03-3.
This article is issued from Wikipedia - version of the 10/11/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.