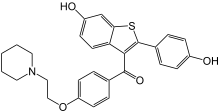
Raloxifene
 | |
| Clinical data | |
|---|---|
| Trade names | Evista |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a698007 |
| License data | |
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | G03XC01 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 2% |
| Protein binding | 95% |
| Metabolism |
Gut glucuronidation[1] CYP system not involved |
| Biological half-life | 27.7 hours |
| Excretion | Fecal |
| Identifiers | |
| |
| CAS Number |
84449-90-1 |
| PubChem (CID) | 5035 |
| IUPHAR/BPS | 2820 |
| DrugBank |
DB00481 |
| ChemSpider |
4859 |
| UNII |
YX9162EO3I |
| ChEBI |
CHEBI:8772 |
| ChEMBL |
CHEMBL81 |
| PDB ligand ID | RAL (PDBe, RCSB PDB) |
| Chemical and physical data | |
| Formula | C28H27NO4S |
| Molar mass | 473.584 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Raloxifene (marketed as Evista by Eli Lilly and Company) is an oral selective estrogen receptor modulator (SERM) that has estrogenic actions on bone and anti-estrogenic actions on the uterus and breast.[2] It is used in the prevention of osteoporosis in postmenopausal women and to reduce the risk of invasive breast cancer in postmenopausal women with osteoporosis and in postmenopausal women at high risk for invasive breast cancer.[3]
Medical use
Raloxifene is indicated for the treatment and prevention of osteoporosis in postmenopausal women. It is also used for reduction of risk and treatment of invasive breast cancer, and it also reduces breast density.[4] For either osteoporosis treatment or prevention, supplemental calcium and/or vitamin D should be added to the diet if daily intake is inadequate.
Adverse reactions
Common adverse events considered to be drug-related were hot flashes and leg cramps.[5]
Raloxifene may infrequently cause serious blood clots to form in the legs, lungs, or eyes. Other reactions experienced include leg swelling/pain, trouble breathing, chest pain, vision changes. Raloxifene is a teratogenic drug, i.e., can cause developmental abnormalities such as birth defects.
Black box warnings were added to the label of raloxifene in 2007 warning of increased risk of death due to stroke for postmenopausal women with documented coronary heart disease or at increased risk for major coronary events, as well as increased risks for deep vein thrombosis and pulmonary embolism.[5]
A report in September 2009 from Health and Human Services' Agency for Healthcare Research and Quality suggests that tamoxifen and raloxifene, used to treat breast cancer, significantly reduce invasive breast cancer in midlife and older women, but also increase the risk of adverse side effects.[6]
A recent human case report in July 2016 suggests that Raloxifene may in fact, at some point, also stimulate breast cancer growth leading to a reduction of advanced breast cancer disease upon the withdrawal of the drug.[7]
Contradications and precautions
Raloxifene is contraindicated in lactating women or women who are or may become pregnant, in women with active or past history of venous thromboembolic events, including deep vein thrombosis, pulmonary embolism, and retinal vein thrombosis and in women known to be hypersensitive to raloxifene.[5]
Pharmacology
The biological actions of raloxifene are largely mediated through binding to estrogen receptors. This binding results in activation of estrogenic pathways in some tissues (agonism) and blockade of estrogenic pathways in others (antagonism). Its agonistic activity at some receptors and its antagonistic activity at others makes it a SERM. Raloxifene appears to act as an estrogen agonist in bone.[5]

Physical and chemical properties
Raloxifene hydrochloride (HCl) has the empirical formula C28H27NO4S•HCl, which corresponds to a molecular weight of 510.05 g/mol. Raloxifene HCl is an off-white to pale-yellow solid that is slightly soluble in water.[5]
Society and culture
An editorial in Lancet Oncology criticized the way that information about the drug was released.[8]
See also
References
- ↑ Jeong, Eun Ju; Liu, Yong; Lin, Huimin; Hu, Ming (2005-03-15). "Species- and Disposition Model-Dependent Metabolism of Raloxifene in Gut and Liver: Role of UGT1A10". Drug Metabolism and Disposition. ASPET. 33 (6): 785–794. doi:10.1124/dmd.104.001883. PMID 15769887. Retrieved 2010-10-20.
- ↑ Muchmore DB (2000). "Raloxifene: A selective estrogen receptor modulator (SERM) with multiple target system effects.". The oncologist. 5 (5): 388–392. doi:10.1634/theoncologist.5-5-388. PMID 11040275.
- ↑ "FDA Approves New Uses for Evista" (Press release). U.S. Food and Drug Administration. 2007-09-14. Retrieved 2007-09-15.
- ↑ Jeon-Hor, Chen; et al. (September 15, 2003). "Reduction of Breast Density Following Tamoxifen Treatment Evaluated by 3-D MRI: Preliminary Study". Magn Reson Imaging. doi:10.1016/j.mri.2010.07.009. Retrieved March 30, 2013.
- 1 2 3 4 5 Raloxifene label Last updated 09/2007]
- ↑ Agency for Healthcare Research and Quality, Rockville, MD. (September 2009). "Medications Effective in Reducing Risk of Breast Cancer But Increase Risk of Adverse Effects". Retrieved 2009-09-14.
- ↑ Lemmo, W (2016). "Anti-Estrogen Withdrawal Effect With Raloxifene? A Case Report". Integrative Cancer Therapies. Published Online Before Print July 13. doi:10.1177/1534735416658954.
- ↑ Thelancetoncology, (2006). "A STARring role for raloxifene?". Lancet Oncol. 7 (6): 443. doi:10.1016/S1470-2045(06)70701-X. PMID 16750489.
External links
- STAR: a head-to-head comparison of tamoxifen and raloxifene as breast-cancer preventatives
- Full Prescribing Information
- Position paper of the National Women's Health Network
- Heringa M (2003). "Review on raloxifene: profile of a selective estrogen receptor modulator". Int J Clin Pharmacol Ther. 41 (8): 331–45. PMID 12940590.
- Barrett-Connor E (2001). "Raloxifene: risks and benefits". Ann N Y Acad Sci. 949: 295–303. doi:10.1111/j.1749-6632.2001.tb04036.x. PMID 11795366.