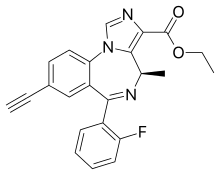
SH-053-R-CH3-2′F
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | 872874-14-1 |
| PubChem (CID) | 11574585 |
| Chemical and physical data | |
| Formula | C23H18FN3O2 |
| Molar mass | 387.406 g/mol |
| 3D model (Jmol) | Interactive image |
| |
SH-053-R-CH3-2′F is a drug used in scientific research which is a benzodiazepine derivative. It produces some of the same effects as other benzodiazepines, but is much more subtype-selective than most other drugs of this class, having high selectivity, binding affinity and efficacy at the α5 subtype of the GABAA receptor. This gives much tighter control of the effects produced, and so while SH-053-R-CH3-2′F retains sedative and anxiolytic effects, it does not cause ataxia at moderate doses.[1] SH-053-R-CH3-2′F also blocks the nootropic effects of the α5-selective inverse agonist PWZ-029, so amnesia is also a likely side effect.[2]
Replacement of the ester function by an amide, realized in the analog MP-III-022, improves selectivity, efficacy and kinetic behavior.[3]
See also
References
- ↑ Savić MM, Huang S, Furtmüller R, Clayton T, Huck S, Obradović DI, Ugresić ND, Sieghart W, Bokonjić DR, Cook JM. Are GABAA receptors containing alpha5 subunits contributing to the sedative properties of benzodiazepine site agonists? Neuropsychopharmacology. 2008 Jan;33(2):332-9. PMID 17392731
- ↑ Savić MM, Clayton T, Furtmüller R, Gavrilović I, Samardzić J, Savić S, Huck S, Sieghart W, Cook JM. PWZ-029, a compound with moderate inverse agonist functional selectivity at GABA(A) receptors containing alpha5 subunits, improves passive, but not active, avoidance learning in rats. Brain Research. 2008 May 7;1208:150-9. doi:10.1016/j.brainres.2008.02.020 PMID 18394590
- ↑ Stamenić TT, Poe MM, Rehman S, Santrač A, Divović B, Scholze P, Ernst M, Cook JM, Savić MM (2016). "Ester to amide substitution improves selectivity, efficacy and kinetic behavior of a benzodiazepine positive modulator of GABAA receptors containing the α5 subunit". Eur. J. Pharmacol. 791: 433–443. doi:10.1016/j.ejphar.2016.09.016. PMID 27639297.